THE NANOPARTICLES, POSSESSING LOW CURIE
TEMPERATURE, AS MEANS
OF SELF – CONTROLLED INDUCTIVE
HEATING
OF TUMOURS.
Bayburtskiy F. S. 1 , 3 , Goncharov L. A. 1 , Korman D. B. 1
, Brusentsov N. A. 1 , 2, Shlyakhtin O. A. 3,
Chekanova A. E. 3, Naletova V. A. 3, Turkov V. A. 3
1.
N. M. Emanuel
Institiute of Biochemical Physics, RAS, Moscow, 119991, Kosygina street.
4. E – mail: Chembio@sky.chph.ras.ru,
Bayburt@mail.ru
2.
N. N. Blokhin
Russian Oncology Research Center, RAMS, Moscow, 115478, Kashyrskoye shosse, 24.
E – mail: Gala752@mail.ru
3.
M. V. Lomonosov Moscow State University,
Moscow, 119899, Vorobyovy gory.
Abstract.
In present article investigation of nanoparticles,
possessing by low Curie temperature, which can be used as self-controlled
mediators in treatment of tumours by a method of magnetic hyperthermia carried
out. Authors had been carried out synthesis of nanoparticles, their physical
and chemical analysis is made and is offered the device allowing qualitatively
and quantitatively to estimate efficiency of a method of magnetic hyperthermia.
Key words: nanoparticles, magnetic fluids, saturation
magnetization, tumour, Curie temperature, magnetic hyperthermia.
Introduction.
Hyperthermia is a rapidly developing technique in cancer therapy. It
takes advantage of the higher sensitivity of tumour tissue to heat and
typically involves heating of the affected organ to 43 – 45°C. Magnetic fluid hyperthermia [1] attracts increasing attention, since
it allows minimizing side effects by the localized heating of only desired
parts of the organism, including tumours located deep inside the patient’s
body. The method involves introduction of ferromagnetic particles (mediators)
into the desired part of the organism and heating them with an alternating
electromagnetic field of radio-frequency range (RF). Magnetic fluids based on
nanocrystalline Fe3O4, stabilized by biocompatible
surfactants [2 – 6], are typically used as mediators. Unfortunately, it is
impossible to control the local temperature near the mediator particles,
possibly causing local overheating and necrosis of normal tissue. This problem
could be solved with ferromagnetic particles of high RF absorption and a Curie
temperature (TC) 42 – 45 °C.
Thus, local temperature control can be ensured even with a nonuniform
distribution of mediator particles throughout the tissue, variable RF intensity
and uneven dissipation of the evolving heat. If TC of this material
can be adapted to the particular medical application.
Similar approach is used in cancer thermal therapies, which utilize
implantable «thermoseeds» with a particular Curie temperature [7,8]. These
macroscopically sized solid units are surgically inserted in the affected organ
and are heated by an external RF. The TC of the «thermoseed»
determines the temperature of the heating. Significant limitations of these
methods are the necessity of a stressful surgical intervention and a relatively
small volume around each «termoseed» which can be effectively heated. The
reduction of size of the heat-producing elements so that they can be delivered
as a suspension via catheter into the bloodstream of the tumour and spread throughout
its capillaries, is the obvious solution of these problems. Sato at al. [9,10]
reported using 50 mm flakes of an amorphous ferromagnetic metal alloy with TC 45°C for intratissue hyperthermia in dogs. However, these relatively large
electrically conducting particles are heated in an RF field by eddy currents even above TC and do not
penetrate into small capillaries. Numerous studies [1 – 6, 12, 13] have shown
that submicron-sized non-conducting particles are most effective for
hyperthermia. The goal of this work was to produce biologically compatible,
electrically non-conductive nanoparticles with TC in this range do
not exist, but by combining several elements and by varying the composition of
the mixture it is possible to produce alloys, amorphous structures, ferrites
and other multi-component system consisting of metals and metalloids. We used
several synthesis technique to produce a variety of ultradisperse particles
with suitable TC and tested their behavior in an RF field.
Materials and methods.
In order to study their RF absorption rate, fine powders of ZnFe2O4
(TC = 100 – 102°C),
La0.8Sr0.2MnO3 (TC = 48°C) and La0.75Sr0.25MnO3 (TC
= 56°C) have been prepared by the freeze-drying synthesis technique [11].
During absorption studies 0.5 g of each powder has been ultrasonically
dispersed in 3 ml of water and placed in a glass test tube inside an air-cooled
inductor (inner diameter 60 mm; length 200 mm) with a matching high-Q-resonator
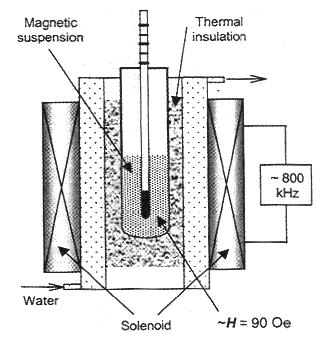
fed with RF power [12] (Figure 1). To avoid RF absorption by metal parts, the
temperature of the fluid was monitored with an alcohol thermometer. A sample of
magnetic fluid containing dextran-coated nanocrystalline Fe3O4
[4,12] was used for comparison. Previous studies have confirmed the absence of
RF absorption in this frequency range by water or by components of the
measuring cell [12].
Results and discussions.
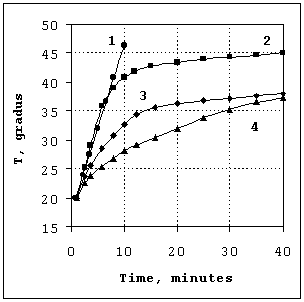
Experimental heating curves are presented in Figure 2. Fe3O4
– based magnetic fluid demonstrates a fast, almost linear heating rate, close
to that shown in [13], without any significant decrease in the tested
experimental conditions. RF irradiation of the ZnFe2O4
suspension leads to significant, but less intense heating with no obvious
temperature limit over the time of the experiment. This curve, taking into account
the lower absorption rate of ZnFe2O4, is also similar to
those of typical magnetic ferrofluids [13]. Both samples of La-Sr manganite
powders demonstrate intense heating during the initial stages of the process,
followed by temperature stabilization at 46.3°C and 37.8°C, respectively. These temperature correspond rather well with the TC
of the samples. The observed difference between TC and the
temperatures of RF absorption termination can be attributed to the sharp
decrease of saturation magnetization MS – usual for ferromagnetics
in the vicinity of TC and predicted by ferromagnetic exchange theory
– and to the heat exchange balance. These results create many prospective
applications of magnetic particles with predetermined TC for heating
of biological tissues and other objects to the optimum temperature using the
demonstrated parametric feedback.
|
Figure 1. Scheme of the experimental setup. |
Figure 2. Time course of the temperature inside the measuring cell
during RF (800 kHz) heating using the following mediators : 1 – Dextran-coated Fe3O4; 2 – La0,75Sr0,25MnO3; 3 – La0,8Sr0,2MnO3; 4 – ZnFe2O4. |
Absorption of RF by ferromagnetic particles of various diameters is
known to involve several physical mechanisms, including different types of
remagnetization processes [5]. Usually RF
absorption strongly depends on the crystallite size. Absorption rates of
superparamagnetic nanoparticles are usually much higher than those of
multi-domain crystallites, mostly due to Neel’s losses. Powders produced by
freeze-drying, are usually substantially agglomerated [14]. Intense milling of
La0.75Sr0.25MnO3 powders in a high-energy
planetary ball mill resulted in a decrease of average aggregate size from 2 to
0.15 – 0.20 microns, as measured by light scattering. This is close to the
average crystallite size observed by SEM (0.1 – 0.2 microns, Figure 3).
Meanwhile, this treatment did not affect the RF absorption rate of this powder,
with the heating curve being practically the same as that of the initial sample
(data not shown). Since the observed size of crystallites even after milling is
still much larger than the typical size of superparamagnetic particles, the
most probable dominating absorption mechanism for these powders is scattering
by displacement of magnetic domain walls. However, RF absorption rates of
polycrystalline manganites are anomalously high compared to those of Fe3O4
polycrystals.
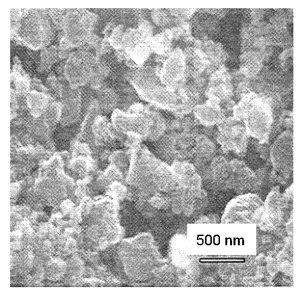
Figure 3. SEM micrograph of La0.75Sr0.25MnO3
powder after milling.
From our results we can conclude, that powders of La-Sr manganites make
it possible to heat a sample by RF to the necessary temperature without
exceeding it. Further heading will be automatically swished off a TLIM ,
which is closely related to TC. Tc of La-Sr manganites strongly depends on the
content of Me in La1 – XMeXMnO3 (where Me =
Sr, Ba, Pb, Ag, Na). By varying the composition of the manganite it is possible
to create materials with TC ranging from 20 to 90°C [15]. Thus , RF heating to any desired temperature in this range
without overheating can be achieved without any external temperature control.
Application of ZnFe2O4 for these purpose is less
attractive due to severe dependence of TC on the metastable, poorly
reproducible cation disrtibution between sub-lattices of the spinel structure,
and a lower absorption rate, while no advantages over existing Fe3O4-based
ferrofluids were observed.
The prospects of a direct manganites application in RF hyperthermia of tumours will depend on the results of the ongoing medical compatibility studies.
Conclusions.
We have proven that the freeze-drying synthesis technique permits the
production of ultradisperse particles with the predetermined composition and TC.
The particles have a high absorption rate at lower temperature and abruptly
stop absorbing RF energy at a particular temperature, preventing further
heating of the sample. We continue searching for more biologically compatible
nanoparticles for self-regulating hyperthermia.
The proposed RF heating with parametric feedback can be useful not only in medicine, but also in solving technological problems, for precise localized temperature control chemical and biochemical reactors. Coating of finely dispersed magnetic particles with a catalyst (including enzymes) combined with RF heating could ensure constant temperature at the reaction zone even in intense mass flows and strongly varying heat supply.
References.
1.
A. Jordan, R. Scholz, R. Felix. // J. Magn. Magn. Mat. 201 (1999) 413 – 419.
2.
R. K. Gilchrist. // Ann. Surgery. 146 (1957) 596
– 606.
3.
M. Shinkai. // J. Magn. Magn. Mat. 194 (1999) 176 – 184.
4.
F. S. Bayburtskiy and N. A. Brusentsov. // J. Magn. Magn. Mat. 194 (1999) 83 – 89.
5.
R. Hergt. // IEEE Trans. Magnetics. 34
(1998) 3745 – 3754.
6.
D. C. F. Chan, D. B. Kirpotin, P. A. // Bull. in Sci. Clin. Appl. Magn. Carriers,
Proc 1st Int. Conf., Plenum, New-York, 1997, P.607 – 618.
7.
J. A. Paulus. // J. Endourology 11 (1997) 295
– 300.
8.
T. C. Cetas, E. J. Gross, Y. Contractor. // IEEE Trans. Biomed. Engineering 45 (1998) 68 – 77.
9.
T. Sato. // IEEE
Trans. Magnetics. 29 (1993) 3325 –
3330.
10. H. Matsuki. // Mater. Sci. and
Engineering A. 182 (1994) 1366 –
1368.
11. O. A. Shlyakhtin, Y. J. Oh, Yu.
D. Tretyakov. // J. Eur. Ceram. Soc. 20
(2000) 2047 – 2054.
12. N. A. Brusentsov and F. S. Bayburtskiy. // J. Magn. Magn. Mat. 225
(2001) 113 – 117.
13. R. Hiergeist. // J. Magn. Magn. Mat. 201 (1999) 420 – 422.
14. Yu. D. Tretyakov, N. N. Oleynikov, O. A. Shlyakhtin. Cryochemical Technology of Advanced
Materials, 1997, Chapman Hall, London.
15. C. N. Rao, R. Mahesh, A. K. Raychaudhuri, R. Mahendiran. // J. Phys.Chem. Solids. 59 (1998) 487 – 502.