SYNTHESIS OF STABLE LYOTROPIC
FERRONEMATICS
WITH HIGH MAGNETIC
CONTENT
V. Berejnov 1,2 , Yu. Raikher 2, V. Cabuil 1, J.-C. Bacri 3, R.
Perzynski 3
1.
Laboratoire de Physicochimie Inorganique,
Université Pierre et Marie Curie, Bat. F, case 63, 4 place Jussieu,
75252 Paris Cedex 05, France.
2.
Laboratory of Kinetics of Complex Fluids,
Institute of Continuous Media Mechanics, Urals Branch of the Russian Academy of
Sciences, Perm, 614013, Russia.
3.
Laboratoire des Milieux
Désordonnés et Hétérogènes,
Université Pierre et Marie Curie, Tour 13, case 78, 4 place Jussieu,
75252 Paris Cedex 05, France
Corresponding
author: Dr.
Valerie Cabuil, Laboratoire de Physicochimie Inorganique, Université
Pierre et Marie Curie, Bat. F, case 63,4 place Jussieu, 75252 Paris Cedex 05,
FRANCE
E-mail: cabuil@ccr.jussieu.fr
Telephone: 33 – 1 – 44 – 27 – 31 – 74
Telefax: 33 – 1 – 44 – 27 – 36 – 75
Abstract.
We report synthesis of colloidal solutions of ferrite
nanoparticles in the surfactant micellar system potassium laurate / 1-decanol /
water within the region of its nematic order. The obtained ferronematic
specimens are reversibly oriented by low (£ 100
Oe) magnetic fields and retain their transparence and stability during several
months.
Key words: liquid crystals, lyotropic nematics, ferrofluids,
orientational transitions
Running
title: Synthesis of lyotropic ferronematics
Introduction.
Introduction of magnetic nanoparticles into
liquid-crystalline matrices is a problem as challenging (1) as it is tricky
(2). About 20 years ago L. Liebert and A. Martinet (3) were the first to
successfully approach it by assigning the role of a matrix to self-assembling
surfactant solutions. They prepared and studied lyotropic ferronematic liquid
crystals resulting from admixing ferrite (magnetite) grains to the ternary
system: sodium dodecylsulphate / 1-decanol / water. The obtained samples proved
to be sufficiently homogeneous and readily orientable by low ( £ 100 Oe) magnetic fields. Despite the tiny amount of the ferrite grains
used (less than 1014/cm3), the lyotropic ferronematics (LFN) of Ref. (3) were inclined to
agglomeration, and the net colloidal stability of the system was rather low. Nevertheless,
due to their remarkable properties, LFN became the objects of interesting
studies (4–7).
In the recent years, the stability of aqueous
dispersions of magnetic nanoparticles has been improved (8). It allowed to
synthesize several other types of magnetic colloids in self-assembling
surfactants, isotropic (magnetic emulsions (9) and vesicles (10,11) or
anisotropic (steric and electrostatic ferrosmectics (12,13) were created and
examined. But the work on the synthesis of LFN did not gain any qualitative
progress since the time of the initial breakthrough (3).
In this letter we report a preparation of LFN by
introducing maghemite nanoparticles into a conventional lyotropic system. The
essential novelties of our systems are: (i)
the particles are present in relatively large amounts (up to 1017/cm3,
that is 1 vol.%) and (ii) the samples
are stable during several months at the least.
Experimental
Materials and methods
The lyotropic system under study is a mixture of
potassium laurate (LK), 1-decanol (dOH) and water. Of those, 1-decanol is
commercially available from FLUKA (purum 98 %). Potassium laurate is
synthesized by ourselves by alcalinization of lauric acid (FLUKA, purum 98%)
with potassium hydroxide (PROLABO, purum 86 %). Lauric acid is dissolved in 0.5
l of water-free ethyl alcohol (PROLABO, purum 99.8%) at 24° C up to the acid concentration ~ 0.25 mol/l. Then water is added to
make the total volume 1 l. The aqueous solution of KOH » 9 mol/l. is added under vigorous stirring until pH of the mixture
reaches 13–14. The resulting transparent solution is cooled down and mixed with
aceton to precipitate the surfactant (LK). After separation, it is dissolved in
water anew, heated up to 100° C and kept boiling for 3 to
6 hours until water evaporates. The emerging well-soluble white powder is once
again dissolved in water at 3–5° C; the pH of the saturated
solution is 12.0. The final purification is carried out by cooling this
solution down to 1°C. The precipitated LK is
separated and dried at room temperature. The resulting powder is soluble in
water at temperatures higher than 5°C,
the pH level at saturation ranges from 10.0 to 10.4.
Magnetic particles are maghemite (g-Fe2O3) grains ~ 6
nm in size, synthesized by alcalinization of aqueous mixtures of ferric and
ferrous salts according to Ref. (8). They are dispersed in water at pH ~ 3 and have positive surface charges. Electrostatic repulsions ensure
the stability of the obtained colloids which are widely known as ionic magnetic fluids or ionic ferrofluids. Being of nanoscopic
dimensions, the particles are subdomain. The magnetization curve of a
ferrofluid is unhysteretic and its analysis allows to determine the particle
size-distribution (14). The volume fraction of the ferrite grains in
ferrofluids as well as in LFN is determined from light absorption measurements
with the accuracy ~ 10 – 3 vol.%.
Synthesis
of LFN specimens
Potassium laurate is mixed with water and then with
1-decanol under stirring at room temperature. The obtained lyotropic is foamed,
and needs centrifugation at 2000–3000 rad/sec to remove the air bubbles. The
reference quantitative composition of the solution on this stage of synthesis
is (wt.%): LK 29.8, dOH 7.2, H2O 63.0. The water content of this
preparation (“empty” matrix) is chosen in such a way that some addition of
water (which will be brought in with the particles) would not cause any
important modifications of the nematic structure, i.e., no borders on the phase
diagram would be crossed. This implies a detailed knowledge of the phase
diagram of the ternary system. Our investigations of this issue are described
elsewhere (Berejnov, V., Cabuil, V., et al. – in preparation), but its main
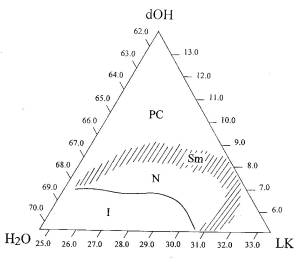
features are shown in Fig. 1. The closed area (N) corresponds to the nematic
phase abutting the isotropic phase (I) from one side and smectic phase (Sm)
from the side of enhanced surfactant concentration. On further growth of the
surfactant contents, the system looses its fluidity and turns into a
polycrystal.
An acidic magnetic fluid (pH 2–3) constituted by
cationic (positively charged) maghemite particles synthesized as indicated
above, is then added drop by drop under vigorous stirring. The mixture is
centrifugated once more, yielding the final product. The content of the latter,
corresponding to the above-given example of the “empty” matrix composition, is
(wt.%): LK 28.1, dOH 6.9, H2O 65.0. The specimens are kept for
several days at rest on a magnet in order to verify that the particles are
indeed incorporated to the nematic supramolecular structure. We consider as LFN
only the specimens which do not segregate under a field gradient ~ 10 T/m.
Results
and discussion
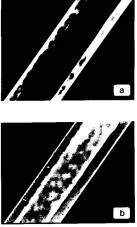
Macroscopically, monophase ferronematics appear as
transparent viscous birefringent fluids of red-brown color (Fig. 2). If they
are put into a flat glass microslide of thickness 0.1 mm (VITRO DYNAMIC Inc.,
used as purchased) and observed under an optical microscope between crossed
polaroids, LFN samples are rather uniformly oriented (Fig. 3a). The samples are uniaxial with a
homeotropic texture with regard to the capillary plane. This texture is
confirmed by the observed defect patterns, if existing.
Application of a magnetic field along the director,
i.e., normal to the capillary plane, leads to an orientational transition with
a threshold H 100 Oe (Berejnov, V., Bacri, J.-C., et al. -- submitted to Europhys. Lett.) – see Fig. 3b. The effect is well reversible: upon
turning off the field, the homeotropic texture restores in a few tens of
seconds.
It appears that a given initial composition of
the nematic (especially, the content of 1-decanol) determines the maximal amount
of particles that it can hold. When exceeding a certain volume fraction,
macroscopic exclusion occurs associated with irreversible precipitation. A
typical composition of a monophase LFN is given in Fig. 3.
By our technique, we obtain homogeneous LFN with the
concentrations up to 1 vol.% (1017 particles per cm3),
that is about 1000 times greater than those reported in Ref. (3). If properly
sealed and kept at constant temperature, they remain unchanged on the time
scale of six months.
We remark that the very fact of existence of the
described ferronematics raises up interesting questions. The main one concerns
the sign of the particle surface charge in our LFN. In the first attempts we
tried to introduce in nematic systems anionic (negatively charged) particles,
since they may be stabilized against agglomeration at the pH level of the
matrix (alcaline medium). However, it appears that only cationic particles,
stabilized usually in the acidic media (pH < 5) yield stable and
concentrated LFN. This result is rather surprising and has to be understood in
order to find out the actual location of the grains relative to the surfactant
micelles.
Conclusions
We describe the method and present the evidence
of successful preparation of lyotropic ferronematic specimens with an enhanced
magnetic content, that was never achieved before. The obtained systems display
high magneto-orientational susceptibility and are rather stable. At the same
time, the problem of determination of their internal structure and field-induced
behavior calls on further investigations.
Acknowledgements
This work was supported by “Le
Réseau Formation – Recherche” № 90R0933 of MENESRIP, by the grant №
96–1149 of the French Direction of Armament(DGA) and from the Russian side by
the grant № 95–02–03953 of RFBR.
References
1. Brochard, F. and de Gennes, P.G., J.
phys. (France) 31, 691 (1970).
2. Rault, J., Cladis, P. E., and Burger, J. P, Phys. Lett. A 32, 199
(1970).
3. Liebert, L. and Martinet, A., J.
phys. Lett. (France) 40, L363
(1979).
4. Liebert, L. and Figueiredo Neto, A. M., J. phys. Lett. (France) 45,
L173 (1984).
5. Figueiredo Neto, A.M., Galerne, Y., Levelut, A.M., and Liebert, L., ibid. 46, L499 (1985).
6. Figueiredo Neto, A. M. and Saba, M. M. F., Phys. Rev. A 34, 3483
(1986).
7. Bacri, J.-C. and Figueiredo Neto, A. M., Phys. Rev. E 50, 3860
(1994).
8.
Bacri, J.-C., Perzynski, R., Salin, D., Cabuil,
V., and Massart, R., J. Magn. Magn. Mater. 85, 27 (1990).
9. Calderon, F.L., Stora, T., Monval, O.M., Poulin, P., and Bibette, J., Phys. Rev. Lett. 72, 2959 (1994).
10. Ménager, C., and Cabuil, V., Colloid
and Polymer Sci. 272, 1299
(1994); J. Colloid Interface Sci. 169, 251 (1995).
11. Bacri, J.-C., Cabuil, V.,
Cebers, A., Menager, C., and Perzynski, R., Europhys.
Lett. 33, 235 (1996).
12. Fabre, P., Cassagrande, C.,
Veyssié, M., Cabuil, V., and Massart, R., Phys. Rev. Lett. 64, 539
(1990).
13. Ménager, C., Cabuil, V., Belloni, L., Dubois, M., and Zemb, Th., Langmuir 12, 3516 (1996).
14. Bacri, J.-C., Perzynski, R., Salin, D., Cabuil, V., and Massart, R., J. Magn. Magn. Mater. 62, 36 (1986).
Figure
Captions
|
Figure 1 Phase diagram of the potassium laurate (LK) / 1-decanol (dOH) / water
system for T=20–22° C and pH = 10.0–10.4. The
regions are marked: isotropic solution (i), nematic phase (N), smectic phase
(Sm), polycrystal (PC); all the concentrations are given in wt.% |
|
|
Figure 2 LFN specimens in tubes (diameter 1 mm) observed between crossed polaroids
with their axes at ± 45° to the vertical direction. The magnetic content in vol.% (from top to
bottom) is: 4.4´ 10–2, 2.2´ 10–1, 1.1. The equivalent (extrapolated to zero particle
content) lyotropic matrix composition (in wt.%) is: LK 26.5, dOH 7.2, H2O
66.3 |
|
|
Figure 3 Micrographs of LFN in a flat microslide (with 1 mm) between crossed
polaroids at ± 45° to its axis; a – no applied
field, homeotropic texture; b –
field ~ 100 Oe normal to the
microslide, planar texture. The lyotropic matrix composition is the same as
in Fig. 2, the magnetic content is 0.022 vol.% |
|