EVALUATION OF
FERROFLUIDS CONTAINING PHOTOSENSITIZER
N. A. Brusentsov 1, A. Yu. Baryshnikov 1, F. S.
Bayburtskiy 2, L. A. Goncharov 2.
1.
Russian Cancer Research Center RAMS, Russian
Federation, Moscow, 115478,
Kashyrskoye shosse, 24, E –
mail: Gala752@mail.ru
2.
Institute of Biochemical Physics RAS, Russian
Federation, Moscow, 119991,
Kosygina street, 4. E –
mail: Bayburt@mail.ru
Abstract.
In present article investigation of cytotoxicity
of a magnetic liquid containing photogem, in a variable magnetic field has been
carried out. By mixture of photogem
(PG) sols with dextran-ferrite (DF) sols PG containing ferromagnetic fluids useful for the tumor
cells PG-magneto-DF-thermosensitization in AC magnetic field were obtained. The
mechanisms of the tumor cells PG-magneto-thermosensitization most likely
involves free-radical processes.
Key words:
nanoparticles, magnetic fluids or ferrofluids, cytotoxicity, photogem, magnetic
field, dextran-ferrite, hyperthermia,
thermosensitization.
Introduction.
We have developed magnetic dextran-ferrite (DF)
nanoparticles [1] and photogem (PG) [2] for tumor cell induction DF magnetic
field hyperthermia (MFH) [3] and PG magneto- [4] and thermosensitization [5]
in the dark (MTS). DF ferrofluids (DFFs), that had been prepared from DF, may
be ideal magnetic carriers [1,3,6]. DF dissipates magnetic field energy and
therefore causes hyperthermia in the area of their confinement [3]. PG in analogy
to haematoporphyrin [4] may generate singled oxygen or superoxide radicals and
cause the destruction of tumor cells in the dark.
The inevitable technical problem of photodynamic
therapy is the initiation of the absorbency of visible light by a tumor that
has been injected with photosensitizing agent, because incident light at
wavelengths between 600 and 1000 nm reacts with the photosensitizing agent only
at shallow depth (0,1 – 1 sm) of tissue.
The purposes of this work were: to evaluate PG-containing
dextran-ferrite ferrofluids for the combination of an MFH with MTS; to analyze
the influence of magnetic field and hyperthermia on cell death and lysis in the
presence of PG; to obtain further insights into the mechanisms of these
processes.
Materials and
methods.
We have tested five water-based dextran-ferrite (DF)
ferrofluids (DFFs): 12.0, 0.6; 0.2; 0.02 and 0.002 %, that were prepared by a
procedure modified from [6]. The sample of the initial DFF was lyophilized. DF
saturation magnetization (MS), other physical and chemical
characterizations and biological
properties were determined as presented in [3, 6]. The analytical fractionation
of the samples of ferromagnetic particles was performed by stepwise passage of
their 1 % aqueous sols through membrane filters (100 nm, 45 nm, 20 nm, XM300,
XM100, XM50, UM20 – UM5u) at a nitrogen pressure of 0,1 – 0,3 atm on an Amicon
TSF-10 thin-channel ultrafiltration system or in Model 12 and Model 202 cells,
and using a column gel-filtration system. Gaussian/Nicomp and volume-weighted
Gaussian distribution analysis of particles in the diluted DFFs and PG sols was
performed by dynamic light scattering laser particle sizing system, Submicron
Particles Sizer NICOMPTM
380/DLS (Particle Sizing Systems, Inc., Santa Barbara, Calif., USA).
PG was obtained treatment of haemin with a 50 %
solution of hydrogen bromide in acetic acid followed by sequentially adding
acetic acid, sodium acetate, and water. Haematoporphyrin diacetate,
precipitated from solution, was filtered and treated with 0,1M sodium hydroxide
for 1 hour. PG was precipitated by acetic followed by filtering, washing with
water, and drying in air. PG prepared as a deep-violet crystal powder
represents a complex half-synthetic mixture of monomeric and oligomeric
porphyrins. The solution of this substance in 0,5 % sodium hydroxide and
adjustment to pH 7.4 with 1 M hydrochloric acid, produced the PG sol.
For biotesting in vitro, two types of tumor cells were
used: adherent human carcinoma ovary (CaOv) and murine ascitic limpholeukosis P388 cells. The latter were
obtained from tumor-bering DBA2 mice on 7th day after intraperitoneal transplantation
of 106 P388 cells. The survival of CaOv and P388 cells, as a result
of exposure to increased temperature and a concentration of DF alone, PG alone,
histidine (His) alone, DF in combination with PG (DF+PG), PG in combination
with His (PG+His) was investigated. Magneto- and thermosensitization of tumor
cells by PG in the dark and heating DFFs achieved simultaneously by an magnetic
field or by flow thermostat. For the CaOv and P388 cell survival study, the
previously used experimental setup [3] was modified. An magnetic field 0.88 MHz, 9.3 kA/m, 0.15 kW
WAS achieved inside a water-cooled copper induction coil of 4.5 sm radius (20
turns with turn-to-turn distance of 0.9 sm). The tumor cells (concentration 106
cells / ml) alone and with reagents: DF, PG, His, PG+DF, PG+His were placed in
the center of the coil and exposed for 30 min to the magnetic field in the
dark. To 6 test tubes (TTs) containing 2 ml of fresh peritoneal ascitic
limpholeukosis P388 or CaOv cells (2x106 / ml) were added respectively:
2 ml of 12 % (w / v) DFF (net g-Fe2O3
weight: 60 mg); 2 ml of 0.6 % DSFF; 2 ml of 0.2 % DFF; 2 ml of 0,02 % DFF; 2 ml
of 0.002 % DFF; and 2 ml of 0.9 % saline to the sixth as a control. The TTs
then were exposed to the magnetic field as described above, and the selected
temperature in the range of 37 to 44°C
was maintained for 30 minutes (Table 1). The cell temperature was measured
during the magnetic field treatment using an alcohol thermometer. Alternatively, the cells were exposed to the
magnetic field in the dark in the presence of PG alone, His alone, PG+DF, and
OPG+His. For the control, the cells were incubated at 37°C in the laboratory thermostat. To 6 isolated TTs were added 0.1 ml of
fresh P388 or CaOv cells (2x106 / ml) and 0.1 ml of reagents as
listed above (Tables 3 – 5); in the sixth (control TT, Tables 1 – 2) 0.1 ml 0.9
% saline was added. The volume of reaction mixture in the TTs, containing tumor
cells and reagents, was 4 ml (Table 1) and 0.2 ml (Tables 2 – 6); the
concentration in all the TTs was 106 cells / ml. The temperature of
the reaction mixtures from 37 to 41°C
was achieved using a flow thermostat (Tables 3 and 5); from 37 to 44°C (Table 1) and from 37 to 41°C
(Tables 4 and 6) was achieved by the magnetic field. The survival of P388 and
CaOv cells as a result of the exposure to concentrations of DF, PG, His, PG+DF
and PG+His at 37 to 41°C
during tumor cell PG magneto- and thermosensitization (MTS) achieved by
magnetic field in the dark was fixed. After CaOv or P388 cells exposure to
magnetic field, DFFs, PG, PG+DF, PG+His at various conditions, the survival of
the cells was analyzed by a haemocytometer counting and by intraperitoneal
injection of 0.1 ml analyzed
compositions of P388 cells to DBA2 mice. The interaction of DF with
the cells was investigated, taking account of the recommendations in [7]. The
results represent the mean ±SD
from the four independent experiments.
Results and
discussion.
DF appeared as dark-brown leaflets and contained about
27 % of g-Fe2O3, 71 % dextran and 2 % H2O; LD50
5 g / kg. 12 % DFF appeared as a dark-brown sol, pH 7,2; MS 1,5 kA /
m, SAR 240 W / g Fe. TEM data allowed
evaluation of the DF particles size: the maximal of the microcrystal and
microsphere diameters were 12 and 240 nm, respectively; in a good accordance
with the results of analytical fractionation and dynamic light scattering
analysis of the DF particle samples and Gaussian / Nicomp and volume-weighted
Gaussian distribution analysis of the [particle diameter in diluted DFFs that
appeared as 2 peaks at 205 and 220 nm (Figure 1).
PG sol particle diameter distribution was in 3 peaks:
peak 1 at 5 to 7 nm; peak 2 at 50 to 70 nm and peak 3 at 300 to 400 nm, and was
in good accordance with PG gel-chromatography results. The obtained DFFs were
resistant to gravitational forces, magnetic fields and liophylising. Determinations
showed direct proportional decrease of MS and heat production to
decrease of DF concentration. Under the chosen conditions, the heating of a
0.9 % NaCl solution was always below the detection limit. DFFs showed
satisfactory heating to 2°C /
mg Fe min. The experimental results are presented in Tables 1 and 4. No
long-term toxicity or acute cell death was detected when cells, were exposed to
DFFs (up to 60 mg DF / ml) alone, or to magnetic field alone for periods of
time up to 6 hours at + 37°C.
Howerer, when P388 or CaOv cells were exposed to MFH at 41 to 44°C for 30 minutes in the presence of DFFs, the high hyperthermia effect
was observed (Table 1).
In the table 1 it is shown: the temperatures in TTs 1 – 6 were proportional to DF concentration: the cell survival fractions at 37 to 41C were high, at 42 to 43°C were insignificant, and at 43 – 44°C were absent. In the table 2 it is shown: PG cytotoxicity obtained by magnetosensitization at 37°C for 30 minutes: the cell lysis and death rate fractions at the low PG concentrations were surpassingly high. In the table 3 it is shown: PG cytotoxicity obtained as a result of CaOv thermosensitization at 41°C for a 30 minutes period. The cell lysis and death fractions were proportional to PG concentration and increased with increasing temperature. The cell survival fractions at 37°C, PG concentration 32.5 mg/ml, were insignificant. In the table 4 it is shown: PG+DF cytotoxicity that were obtained at 41 to 43°C and at 37°C for a 30 minutes period; the cell lysis and death fractions were proportional to the concentrations of PG and DF. As the result of combination MFH with MTS at the moderate concentrations of PG (0.8 – 32.5 mg/ml) and the high concentrations of DF (3 – 9 mg/ml) the cell survival fraction was absent; at the moderate PG concentrations (3.25 – 32.5 mg/ml) and the low DF concentrations (0.001 – 0.1 mg/ml) the cell survival fractions ere average; at the high PG concentration (325 mg/ml) and the low DF concentration the cell survival fraction was absent. In the table 5 it is shown: PGF cytotoxicity that was obtained by the incubation of 388 cells at 41°C and 37°C for a 30 minutes period, the cell lysis and death fractions were proportional to the concentrations of PG and temperature. As a result of hyperthermia at 41°C for 30 minutes with PG thermosensitization of P388 cells at concentrations of PG (0.3256 – 32.5 mg/ml) the cell survival fractions were proportional to the concentrations of PG; at 37°C and the same PG concentrations the cell survival fractions were three times as high. In the table 6 it is shown: that substantial inhibition of cell lysis and death by PG in the presence of 1.6 mg/ml His was observed. Magnetic field cell damage enhancement by PG at 37°C (test tube 2) and simultaneous thermal- and MFH magnetic field cell damage enhancements by PG at 41°C (test tube 5) was effectively suppressed by the addition of singled oxygen scavenger, His (TTs 3,6).
We investigated the role of: DF, PG, His, PG+DF, PG+His alone; hyperthermia, Magnetic field induction, DF magnetic field hyperthermia alone; PG magneto- and thermosensitization in the dark (MTS) to increase the destruction of tumor cells. Two types of tumor cells: adherent human carcinoma ovary (CaOv) and murine ascitic limpholeucosis P388 cells were incubated in the presence or absence of the enumerated reagents and physical factors. The cells were successively heated at 41 to 44°C by magnetic field treatment with the 0.88MHz, 9.3 kA/m, 0.15 kW induction coil. The combined effects of MFH and MTS were then examined and tested for statistical significance. Significant differences between cytotoxic effects produced by MFH, MTS and the combination of MFH with MTS were found. PG at nontoxic doses at 37°C significantly enhancement by PG was effectively suppressed by the addition of His, singlet oxygen and a superoxide scavenger. In the presence of PG+His non-toxic doses, the cell survival fractions were proportional to the temperature. Significant differences between cytotoxic effects produced by PG at 37°C and 41°C at the same concentrations of PG were found. Therefore the cytotoxicity of MFH should be attributed to the effects of heat itself. Combination of PG with DF have potential as a magneto- and thermosensitizer because of the following advantages: their dose-dependent enhancement of magneto- and thermal cell damage; lack of toxicity at physiological parameters, magnetic field (frequency, induction, strength, power and temperature), and at the non-toxic doses of PG+DF required for tumor cell magneto- and thermosensitization. Combination of MFH with MTS is the summary method. These data confirm the feasibility of using induction DF magnetic field hypertermia in combination with tumor cell PG magneto- and thermosensitization. The advantage of this method is the much deeper penetration of the magnetic field into body tissues as compared to light. Further in vitro and in vivo investigations allow choosing of the PG+DF optimal doses and magnetic field range intensity and continuity.
Conclusion.
Dissolution of dextran-ferrite in water results in formation of dextran-ferrite ferrofluids useful for the magnetically controlled combination of magnetic field induced hyperthermia with photogem magneto- and thermosensitization of tumor cells. The mechanism of ferrimagnetic heating most likely involves the magnetization relaxation loss process, and tumor cells photogem magneto- and thermosensitization most likely involves free-radical processes with a key role of superoxide radical.
References.
1. Autenshlyus A. I., Brusentsov N. A., Lockshin A. // J. Magn. Mat. Mat., 122 (1993), 360 – 363.
2. Mironov A. F., Nizhnik A. N., Nockel A. Yu. // J. Photochem. Photobiol., 4 (1990), 297 – 306.
3. Brusentsov N. A., Gogosov V. V., Brusentsova T. N., Sergeev A. V., Yurchenko N. Yu., Shumakov L. I. // J. Magn. Mat. Mat., 225 (2001), 113 – 117.
4. Babincova M., Babinec P., Leszchinska D., Sourivong P. // J. Magn. Mat. Mat., 225 (2001), 194 – 196.
5. Saito A., Tanaka R., Takahashi H., Kakimura K. // Int. J. Hyperethermia, 14 (1998), 503 – 511.
6. Brusentsov N. A., Yurchenko N. Yu., Osipov N. E., Bayburtskiy F. S. // J. Magn. Mat. Mat., 194 (1999), 83 – 89.
7. Hafeli U. O., Pauer G. J. // J. Magn. Mat. Mat.,194 (1999), 76 – 82.
The
figure and tables:
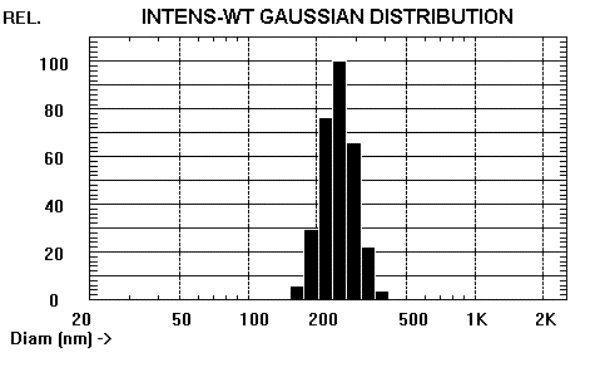
Figure 1. Particle sizing
analysis of DFF
Table I. Influence of DF on CaOv cells
during 30 min of magnetic field exposure.
|
Test tubes |
Survival cells, % |
Dead cells, % |
DF, mg / ml |
T,°C |
|
1 |
0.0 |
100±6 |
60.0 |
43 – 44 |
|
2 |
4±0.8 |
96±5.8 |
6.0 |
42 – 43 |
|
3 |
48±3.4 |
52±3.6 |
1.00 |
41 – 42 |
|
4 |
91±5.5 |
9±1.4 |
0.10 |
39 – 40 |
|
5 |
95±5.8 |
5±1.0 |
0.01 |
37 – 38 |
|
6 |
96±5.8 |
4±0.8 |
0.00 |
37 |
Table 2.
Influence of PG on CaOv cells during 30 min of magnetic field exposure at 37°C.
|
Test tubes |
Survival cells, % |
Dead cells, % |
Cells lysis, % |
PG (mg / ml) |
|
1 |
0.0 |
0.0 |
100±6 |
325.00 |
|
2 |
9.5±1.5 |
60±4.0 |
30.5±2.5 |
32.50 |
|
3 |
54±3.7 |
28±2.5 |
18±1.9 |
3.25 |
|
4 |
87.3±5.5 |
5.7±1.0 |
7.0±1.3 |
0.65 |
|
5 |
91±5.5 |
5.0±1.0 |
4.0±0.8 |
0.06 |
|
6 |
95±5.8 |
4.0±0.8 |
1.0±0.1 |
0.00 |
Table 3.
Influence of PG on CaOv cells during 30 min exposure in the flow thermostat at
37°C (TTs 1
– 3) and at 41°C (TTs 4 – 6).
|
Test tubes |
Survival cells, % |
Dead cells, % |
Cells lysis, % |
PG (mg / ml) |
|
1 |
3.0±0.6 |
6.0±1.1 |
91.0±5.5 |
325.00 |
|
2 |
29.0±2.5 |
50.0±3.5 |
21.0±2.0 |
32.50 |
|
3 |
66.0±4.3 |
16.0±1.8 |
18.0±2.4 |
3.25 |
|
4 |
10.0±1.5 |
55.0±3.8 |
35.0±2.8 |
32.5 |
|
5 |
38.0±2.9 |
39.0±3.0 |
23.0±2.2 |
3.25 |
|
6 |
82.0±5.1 |
14.0±1.7 |
4.0±0.8 |
0.32 |
Table 4.
Influence of DF and PG on CaOv cells during 30 min of magnetic field
exposure at 41 to 43°C (TTs 1 – 3) and at 37°C (TTs 4 – 6).
|
Test tubes |
Survival cells, % |
Dead cells, % |
Cells lysis, % |
DF, mg / ml |
PG (mg / ml) |
|
1 |
0.0 |
91.0±5.5 |
9.0±1.4 |
9.0 |
0.80 |
|
2 |
0.0 |
88.0±5.4 |
12.0±1.6 |
6.0 |
1.60 |
|
3 |
3.0±1.0 |
52.0±3.6 |
45.0±3.3 |
3.0 |
32.50 |
|
4 |
20.0±2.0 |
45.0±3.3 |
35.0±2.8 |
0.10 |
3.25 |
|
5 |
10.0±1.0 |
50.0±3.5 |
40.0±3.0 |
0.01 |
32.50 |
|
6 |
0.0 |
0.0 |
100.0±6.0 |
0.001 |
325.0 |
Table 5.
Influence of PG on P388 cells during 30 min flow thermostat exposure
at + 41°C (test tubes 1 – 3) and at + 37°C (test tubes 4 – 6).
|
Test tubes |
Survival cells, % |
Dead cells, % |
Cells lysis, % |
PG (mg / ml) |
|
1 |
9.0±1.4 |
54.0±3.7 |
37.0±2.9 |
32.5 |
|
2 |
39.0±3.0 |
36.0±2.8 |
25.0±2.3 |
3.25 |
|
3 |
70.0±4.5 |
18.0±1.9 |
12.0±1.6 |
0.325 |
|
4 |
29.0±2.5 |
40.0±3.0 |
31.0±2.5 |
32.5 |
|
5 |
88.0±5.4 |
8.0±1.2 |
4.0±0.8 |
3.25 |
|
6 |
91.0±5.5 |
5.0±1.0 |
4.0±0.8 |
0.325 |
Table 6.
Influence of PG and His on P388 cells during 30 min of magnetic field exposure
at 37°C (test tubes 1 – 3 ) and at 41°C (test tubes 4 – 6).
|
Test tubes |
Survival cells, % |
Dead cells, % |
Cells lysis, % |
PG (mg / ml) |
His, mg / ml |
|
1 |
94.0±5.7 |
6.0±1.2 |
0.0 |
0.0 |
1.6 |
|
2 |
9.0±1.4 |
51.0±3.5 |
40.0±3.0 |
32.5 |
0.0 |
|
3 |
77.0±4.9 |
12.0±1.6 |
11.0±1.5 |
32.5 |
1.6 |
|
4 |
84.0±5.2 |
16.0±1.8 |
0.0 |
0.0 |
1.6 |
|
5 |
3.0±0.6 |
51.0±3.5 |
46.0±3.3 |
32.5 |
0.0 |
|
6 |
63.0±4.2 |
20.0±2.0 |
17.0±1.8 |
32.5 |
1.6 |