CALCULATION OF THE PAIR POTENTIAL INTERACTION
IN
ELECTRIC DOUBLE LAYERED
MAGNETIC FLUIDS :
A
QUANTITATIVE ANALYSIS OF
THE pH - DEPENDENT PHASE DIAGRAM.
A. F. C. Campos 1 , F. A. Tourinho 1 , G. J. Da
Silva 2 , J. Depeyrot 2 .
1.
Complex Fluids Group, Instituto de
Quimica, Universidade de Brasilia, Brazil.
2.
Complex Fluids Group, Instituto de
Fisica, Universidade de Brasilia, Brazil.
One of the major challenges in the research of
electrostatic stabilized colloidal dispersions is to predict the stability
conditions of the system from theoretical data coupled with experimental measurements.
In the case of electric double layered magnetic fluids (EDL-MF), besides the
balance between the Van Der Waals attraction and the screened electrostatic repulsion
(DLVO potential), the attractive magnetic dipolar interaction has to be
included to control the thermodynamical stability of the dispersion. The
interplay between these three contributions leads to a pair potential interaction
function which exhibits a primary minimum at short distances, a positive energy
barrier at intermediate distances and a secondary minimum at large distances.
At constant temperature and in absence of external magnetic field,
modifications of the ionic strength and particle charge may induce phase
transitions [1]. If the energy barrier is sufficient low or inexistent, the
particles aggregate in the primary minimum of their potential energy
(coagulation). In this irreversible phase transition, the particles are held
together by strong Van Der Waals forces and do not break apart without strong
external forces. When both an energy barrier and a secondary attractive minimum
energy exist, the particles may aggregate at this minimum and the system become
kinetically stable. Nevertheless, it as a weaker effect and a reversible
transition (flocculation).
Simultaneous potentiometric and conductometric
titrations have been used as a powerful tool to determine the particle charge
in EDL-MF [1, 2]. It has been evidenced that the EDL-MF systems inductive as a
mixture of strong acid and weak diprotic, corresponding to the bulk dispersion
and particle surface, respectively. The particle superficial density of charge
is generated through the equation
reactions of superficial metal ions which undergo hydrolysis, leading to three
kind of superficial sites: positively (negatively) charged in acidic (basic)
medium and discharged in neutral one. The analysis of equilibrium involved
between the particle surface and the bulk dispersion allows to determine the
pH dependence of the surface charge density. As the hydrogen ionic concentration
of the EDL-MF dispersion controls the particle charge [1], the interactions
between nanoparticles also may be tuned varying the pH. Recently, the
stability of EDL-MF based on 7.1 nm mean sized maghemite particles has been
investigated as function of pH at constant ionic strength and at room
temperature [4]. It has been observed a thixotropic gel phase between the sol
one and the coagulation zone in pH regions between 3.9 and 4.9 in acidic medium
and 9.6 and 10.8 in basic one. In the present work two fundamental targets are
focused. The first one is to establish the experimental pH-dependent phase
diagram of an acid EDL-MF sample based on manganese ferrite nanoparticles of
mean size around 7.5 nm. The later is to calculate the pair potential
interaction of the nanosized particles in the framwork of the extended DLVO
theory in order to improve the understanding of the pH-dependent phase
diagram.
The EDL-MF sample was synthesized by following the
procedures described elsewhere [4]. In a first step, it has been performed a
hydrothermal coprecipitation of aqueous solutions of MnCl2 – FeCl3
in alkaline medium. Then the particles were conveniently peptized in acidic
medium by adjustment of the ionic strength. The resulting dispersions are
stable sols of high quality. The phase diagram was built up as follows: for a
volume fractions j = 1.8 %, many samples of the
precursor ferrofluid dispersion at different pH (2.0 £ pH £ 7.5) were prepared, pH being adjusted by addition of varying quantities
of tetramethylammonium hydroxide (TAMOH). In order to determine the pH
dependence of the surface charge density, simultaneous potentiometric titration
of 40 ml of the ferrofluid sample (volume fraction j = 1.5 % corresponding to approximately 6.8 x 1021
particles per m3) were performed using titrant solutions of sodium
hydroxide 0.099 mol x l – 1 with an electronic burette Metrohm 715
DOSIMAT. The potentiometric readings were carried out with a pH – meter Metrohm
713 using a pH glass double-junction electrode, which includes a salt bright in
order to avoid the direct contact of the colloidal solution with the glass
membrane. The conductivity k was measured with a conductometer Metrohm 712 using an imersion-type
measuring cell. All reagents used in this work are of analytical grade from
Aldrich or Merck. The saturation value of the surface charge density was found
equal to 0.25 Cm – 2 which approximately corresponds to one charge
per 0.64 nm2 or about 276 sites per particle. This value is in
excellent agreement with the the reported ones [5]. Figure 1 exhibits the
pH-dependent phase diagram of the EDL-MF sample and the pH ranges of the phase transitions observed are
similar to the reference [4].
Moreover, the height of the energy barrier (W) obtained from the pair potential
calculation as the variation of the surface charge density (s0) are plotted as
a function of pH. The electrostatic contribution to the interaction potential
is achieved by the expansion of the Poisson-Boltsmann equation up to cubic
terms [6]. In low pH medium, the sol state is ensured by a high energy barrier
(22 kBT £ W £ 18 kBT) related to
the saturation value of the surface charge density. With increasing pH, it is
observed a reduction of the energy barrier and a superficial density of charge
decrease about 50 % of its saturation value. These conditions leads to a
reversible place transition (flocculation) generating a thixotropic gel state
of kinetic stability. For pH ³ 5.2 it acidic medium, W and s0 fall drastically so that the
thermal fluctuations are sufficient to include primary minimum aggregation
(coagulation).
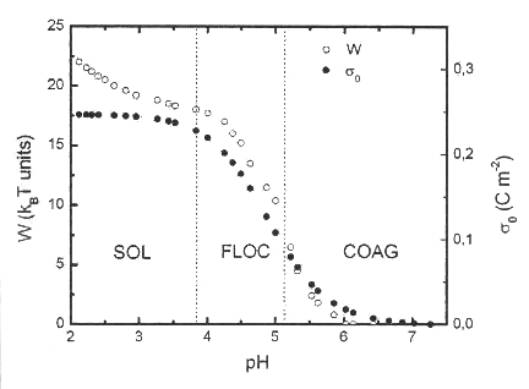
Figure 1.
The pH-dependent phase diagram of the EDL-MF sample, the height
of the energy barrier (W) and the variation of the surface charge
density s0 with pH.
References:
1.
J. N. Israelachvili, Intermolecular
and Surface Forces (Academic Press, New York, 1985). J.- C. Bacri, R.
Perzynski, D. Salin, V. Cabuil, R. Massart. // J. Colloid and Interfaces
Science, 132 (1983) 43.
2.
F. A. Tourinho, A. F. C. Campos, R.
Aquino, M. C. F. I. Lara, J. Depeyrot. // Braz. J. Phys. 32 (28) (2202) 501.
3.
A. F. C. Campos, F. A. Tourinho, G.
J. Da Silva, M. C. F. I. Lara, J. Depeyrot. // Eur. Phys. J. E 4 (2001) 25.
4.
E. Hasmonay, A. Bee, J. – C. Bacri,
R. Perzynski. // J.. Phys. Chem. B 103
(1999) 6421.
5.
F. A. Tourinho, R. Franck, R.
Massart. // J. Mater. Sci. 25 (1990)
3249.
6.
A. O. Ivanov // Colloidal Journal 59 (1997) 446.