Performance of dye-affinity
beads for aluminium removal in magnetically stabilized fluidized bed
Handan Yavuz*1, Ridvan Say2,
Miige Andac1, Necmi Bayraktar3 and Adil Denizli1
Address:
1 Department of Chemistry, Biochemistry
Division, Hacettepe University, Ankara, Turkey, 2Department of
Chemistry, Anadolu University, Ankara, Turkey and
3Faculty of Medicine, Urology Department,
Hacettepe University, Ankara, Turkey
Email: Handan Yavuz* - handany@hacettepe.edu.tr;
Ridvan Say - rsay@anadolu.edu.tr; Mtige Andac - mugeandac@yahoo.com; Necmi
Bayraktar - necmibayraktar@hotmail.com; Adil Denizli - denizli@hacettepe.edu.tr
* Corresponding author
Abstract
Background: Aluminum has recently been recognized as a
causative agent in dialysis encephalopathy, osteodystrophy, and microcytic
anemia occurring in patients with chronic renal failure who undergo long-term
hemodialysis. Only a small amount of Al(III) in dialysis solutions may give
rise to these disorders.
Methods: Magnetic poly(2-hydroxyethyl methacrylate)
(mPHEMA) beads in the size range of 80-120 цт were produced by free radical
co-polymerization of HEMA and ethylene dimethacrylate (EDMA) in the presence of
magnetite particles (Fe3O4). Then, metal complexing
ligand alizarin yellow was covalently attached onto mPHEMA beads. Alizarin
yellow loading was 208 |imol/g. These beads were used for the removal of
Al(III) ions from tap and dialysis water in a magnetically stabilized fluidized
bed.
Results: Al(III) adsorption capacity of the beads
decreased with an increase in the flow-rate. The maximum Al(III) adsorption was
observed at pH 5.0. Comparison of batch and magnetically stabilized fluidized
bed (MSFB) maximum capacities determined using Langmuir isotherms showed that
dynamic capacity (17.5 mg/g) was somewhat higher than the batch capacity (1 1.8
mg/g). The dissociation constants for Al(III) were determined using the
Langmuir isotherm equation to be 27.3 mM (MSFB) and 6.7 mM (batch system),
indicating medium affinity, which was typical for pseudospecific affinity ligands.
Al(III) ions could be repeatedly adsorbed and desorbed with these beads without
noticeable loss in their Al(III) adsorption capacity.
Conclusions: Adsorption of Al(III) demonstrate the
affinity of magnetic dye-affinity beads. The MSFB experiments allowed us to
conclude that this inexpensive sorbent system may be an important alternative
to the existing adsorbents in the removal of aluminium.
Background
About 8% of the Earth's crust is comprised of
aluminium. This element is the most abundant metal naturally present in air,
soil and water. Consequently, environmental exposure to aluminium is
potentially possible. Its ingestion is unavoidable since aluminium compounds
are added not only to most water supplies but also to many processed foods and
medicines. Aluminium is a known neurotoxi-cant. It enters the brain, where it
contributes to some neuro-degenerative diseases including dialysis
encepha-lopathy, osteomalacia, osteodystrophy, in particular those related to
dialysis treatment of uremic subjects [ 1 ]. Only a small amount of Al(III)
ions in dialysis solutions may cause these disorders. Aluminium may contribute
to Alzheimer's disease [2]. Aluminium is also able to give rise to toxicity in
the bones and hematopoietic system in humans [3].
Positively charged aqua and hydroxy-monomeric forms
have been found to be the most toxic species of aluminium to living organisms
in the terrestrial and aquatic environments [4]. Generally, aluminium sulphate
is used as a coagulant in the treatment of water to help the removal of
suspended matter and highly coloured humic substances [5], thus reducing the
dose of chlorine later required to ensure satisfactory microbiological quality.
Hence, potable water often contains high aluminium levels of natural origin
and/or from the water purification process [6].
The selective removal of aluminium ions have been
extensively investigated by applying several techniques [7-9]. Among them, the
use of specific polymeric adsorbents has been considered as one of the most
promising techniques [10,11]. Specific adsorbents consist of a ligand (e.g.,
reactive textile dye, ion-exchange functional groups or chelat-ing agents)
which interacts with the metal ions specifically, and a carrier solid matrix.
There have been several separation approaches
performed under magnetic field [12]. The most well known technique is the
magnetically stabilized fluidized bed. Magnetically stabilized fluidized bed
exhibits combination of the best characteristics of both packed and fluidized
bed. These include the efficient fluid-solid mass transfer properties,
elimination of particle mixing, low pressure drop, high feed-stream solid
tolerances, good fluid-solid contact, elimination of clogging and continuous
countercur-rent operation [13]. Especially, when dealing with highly viscous
mediums contact with the magnetic adsorbent in a magnetically stabilized
fluidized bed is desirable because of high convective transport rates.
Recently, there has been increased interest in the use of magnetic adsorbents
in biomolecule coupling and nucleic acid purification [14]. Magnetic
adsorbents can be produced using inorganic materials or polymers. High
mechanical resistance, insolubility and excellent shelf life make inorganic
materials ideal as adsorbent. The main disadvantage of inorganic supports is
their limited functional groups for ligand coupling. Magnetic adsorbents can be
porous or non-porous [15]. They are more commonly manufactured from polymers
since they have a variety of surface functional groups which can be tailored to
use in different applications [16-22].
In the present study, we attempted to use alizarin
yellow-attached magnetic poly(2-hydroxyethyl methacrylate) (mPHEMA) beads as
specific adsorbent for aluminium removal from aqueous solutions in a magnetically
stabilized fluidized bed. Al(III) adsorption on the alizarin yellow-affinity
beads from aqueous solutions containing different amounts of Al(III) ions and
at different pHs is reported here. Finally, reuse of the dye-affinity beads is
also discussed.
Materials and methods
Materials
2-hydroxyethyl methacrylate (HEMA), was purchased from
Sigma (St. Louis, MO, USA), and was purified by vacuum distillation under a
nitrogen atmosphere. The comonomer, ethylene dimethacrylate (EDMA, Merck,
Darmstadt, Germany) was used as the crosslinking agent. Magnetite particles (Fe3O4,
diameter < 1 цт) were obtained from Aldrich (USA).
Alizarin yellow (3,4-dihy-droxy-9,10-dioxo-2-anthracenesulfonic acid, sodium
salt mono-hydrate) was purchased from BDH (Poole, UK). All other chemicals were
obtained from Merck as analytical grade. All water used in the adsorption
experiments was purified using a Barnstead (Dubuque, IA) ROpure LP® reverse
osmosis unit with a high flow cellulose acetate membrane (Barnstead D2731)
followed by a Barnstead D3804 NANOpure® organic/colloid removal and ion
exchange packed bed system.
Preparation of magnetic
PHEMA beads
Details of the preparation and characterization of the
mPHEMA beads were reported elsewhere [23]. The mPHEMA beads were prepared by
suspension polymerization. A typical suspension copolymerization procedure of
mPHEMA beads was performed as below: The dispersion medium was prepared by
dissolving 200 mg of poly(vinyl alcohol) (PVA; molecular weight: 50.000) within
50 ml of distilled water. The desired amount of 2,2'-azobisisobutyronitrile
(AIBN) (0.06 g) was dissolved within the monomer phase 12.0/4.0/8.0 ml (EDMA/
HEMA/toluene) with 1.0 g magnetite particles. This solution was then
transferred into the dispersion medium placed in a magnetically stirred (at a
constant stirring rate of 600 rpm) glass polymerization reactor (100 ml) which
was in a thermostatic water bath. The reactor was flushed by bubbling nitrogen
and then was sealed. The reactor temperature was kept at 65° C for 4 h. The
temperature was then raised to 90°C and kept constant by a ther-mostated water
bath during the polymerization time (2 h). After polymerization, the mPHEMA
beads were separated from the polymerization medium. The residuals (e.g., unconverted monomer,
initiator and other ingredients) were removed by a
cleaning procedure. Briefly, beads were transferred into a reservoir, and washing
solutions (i.e., a dilute HCI solution, and a water-eth-anol mixture) were
recirculated through the system which includes also an activated carbon column,
to be assured that the magnetic beads were clean. Purity of the magnetic beads
was followed by observing the change of optical densities of the samples [X:
280 nm) taken from the liquid phase in the recirculation system, and also from
the DSC thermograms of the magnetic beads obtained by using a differential
scanning microcalorimeter (Mettler, Switzerland). Optical density of uncleaned
magnetic beads was 2.63, but after the cleaning operation this value was reduced
to zero. In addition, when the thermogram of uncleaned beads was recorded, it
had a peak around 60° С This peak might originate from AIBN, but
after application of the cleaning procedure, no peak between 30-100° C was
observed on the thermogram.
The dry density of the magnetic beads was measured
with pycnometer by dispersing the dry beads in ethanol.
Alizarin yellow
attachment
Preparation and characterization of the alizarin
yellow-attached mPHEMA beads were reported in our previous paper in detail
[24]. In order to prepare the alizarin yellow-attached magnetic beads
following procedure was applied. 5.0 g of dry magnetic beads was weighed and
transferred into the SOCl2 (Carlo Erba, Italy) (10 ml). This
reaction medium was boiled in rotary evaporator for 6 h. Then, 2.5 g alizarin
yellow was dissolved in absolute ethanol (30 ml). Alizarin yellow-attachment
process was performed in ethanol solution for 24 h. At the end of this
reaction period, the alizarin yellow-attached beads were removed by filtration
and washed with ethanol, water and tetrahydrofuran several times until all the
unbound dye molecules were removed. The dye attached beads were stored at 4°C
with 0.02% sodium azide to prevent micro-bial growth.
The leakage of the alizarin yellow from the
dye-attached beads was investigated within the media at the selected pH in the
range of 2.0-7.0. These media were the same which were used in the Al(III)
adsorption experiments. The medium with the dye attached magnetic beads was
stirred for 24 h at room temperature. Then, magnetic beads were separated from
the medium, and the alizarin yellow concentration was measured in the liquid
phase by spectrophotometry at 500 nm.
Magnetically stabilized
fluidized bed procedure
Al(III) adsorption studies were carried out in a
magnetically stabilized fluidized bed. Beads suspended in pure water were
degassed under reduced pressure (by using water suction pump) and magnetically
stabilized into a column (10 cm x 0.9 cm inside diameter) equipped with a water
jacket for temperature control. The vertically oriented magnetic field was
produced by passing DC current through two modified Helmholtz coils (1.5 cm
diameter x 2.5 cm thick) spaced 4 cm apart. At a current of 1.6 A (50 W), each
coil produced a magnetic field of 40 Gauss. Equilibration of the column was
performed by passing four column volumes of phosphate buffer (pH: 7.4) before
injection of the Al(III) solution. In a typical adsorption system, 50 ml of the
aqueous Al(III) solution was passed through the column containing magnetic
beads, by a peristaltic pump for 2 h. After loading, the column was washed
with deionized water to wash out Al(III) impurities. The concentrations of the
Al(III) ions in the aqueous phases after the desired treatment periods were
measured by using a graphite furnace atomic absorption spectro-photometer (AAS
5EA, Carl Zeiss Technology, Zeiss Analytical Systems, Germany). Deuterium
background correction was used. Pyrolitic graphite coated tubes were used for
AAS measurements. The instrument response was periodically checked with known
Al(III) solution standards. The experiments were performed in replicates of
three and the samples were analyzed in replicates of three as well. For each
set of data present, standard statistical methods were used to determine the
mean values and standard deviations. Confidence intervals of 95% were
calculated for each set of samples in order to determine the margin of error.
In the first group of experiments, the flow rate of
the aqueous solution (i.e., 50 ml of the solution with a Al(III) content of
50 mg/L) was changed between 0.5-3.0 mL/min. In the second group of
experiments, Al(III) adsorption from aqueous solution was studied at different
pH's (2.0-7.0). Adsorption isotherm was also obtained in the magnetically
stabilized fluidized bed. Aqueous solutions containing different amount of
Al(III) were used in these experiments. The changes in the Al(III)
concentration with time was followed to obtain the adsorption curves. The
amount of adsorbed Al(III) per dry magnetic beads was calculated by using the
concentrations of the Al(III) in the initial solution and in the equilibrium.
Desorption and repeated
use
In all cases adsorbed Al(III) ions were desorbed using
0.1 M HNO3 solution. In a typical desorption experiment, 50 ml of
the desorption agent was recirculated through the magnetically stabilized
fluidized bed containing dye-affinity magnetic beads for 1 h. The
concentrations of the Al(III) ions in the desorption medium were measured by
using a graphite furnace atomic absorption spectropho-tometer. The desorption
ratio was calculated from the amount of Al(III) adsorbed on the magnetic beads
and the final Al(III) concentration in the desorption medium. In order to test
the reusability of the dye-affinity magnetic beads, Al(III)
adsorption-desorption procedure was repeated ten times by using the same
magnetically stabilized fluidized bed.
Batch procedure
Adsorption of Al(III) from aqueous solution was also
investigated in batch experiments. Aqueous Al(III) solution (50 ml) was
treated with the magnetic dye-affinity beads at room temperature, in the flasks
agitated magnetically at an agitation speed of 600 rpm for 2 h. The suspension
was brought to pH 5.0 by adding sodium hydroxide and nitric acid. The pH was
maintained in a range of ± 0.1 units until equilibrium was attained. Polymer
amount was kept constant at 100 mg per 50 ml. Al(III) determination was
performed in water sample in an atomic absorption spectrophotometer coupled to
a graphite furnace atomiser. Adsorption values (mg/g) were calculated as the
difference in Al(III) ion concentration of the pre- and post adsorption
solutions divided by the weight of dry magnetic affinity beads.
Results and discussion
Characteristics of mPHEMA
beads
mPHEMA beads (in the size range of 80-120 |im) carrying
alizarin yellow were prepared as a specific affinity adsorbent for removal of
Al(III) from the water which was used for preparation of dialysis solution.
mPHEMA beads used in this study were prepared and characterized in our earlier
study [24]. The main criteria of selection of PHEMA is due to its mechanical
strength and chemical stability. With the goal of testing the mechanical
stability of the magnetic beads, a sample of these magnetic beads was treated
in a ball mill for 60 min. Negligible percentage of the sample was broken. The
dry density of the magnetic beads was measured as 1.27 g/cm3. The
magnetic beads are crosslinked hydrogels. They do not dissolve in aqueous
media, but do swell, depending on the degree of cross-linking and on the
hydrophilicity of the matrix. The equilibrium swelling ratio (the ratio of the
volumes of the microbeads before and after swelling) of the beads used in this
study is 34%. The simple incorporation of water weaken the secondary bonds
within the hydrogels. This enlarges the distance between the polymer chains and
causes the uptake of water. It should be mentioned that the water uptake
properties of the mPHEMA beads did not change after Alizarin Yellow attachment.
After the attachment of the dye (i.e., alizarin
yellow) the size of the swollen beads did not change, but the colour became
dark yellow, which is a clear indication of the incorporation of the dye
molecules in the structure of the mPHEMA microbeads. As shown in our previous
paper, the dye molecules were attached to the mPHEMA beads by covalent bonding
via hydroxyl groups [24]. The mPHEMA beads containing 208 |imol alizarin
yellow/g
polymer, which was the maximum amount of dye-attachment
that we have reached, were used in this study. Alizarin Yellow release from
the mPHEMA beads was also monitored continuously. There were no dye release in
any of the adsorption and desorption media, which assured that the cleaning
procedure used for removal of physically adsorbed alizarin yellow molecules
from the mPHEMA beads was satisfactory.
Column performance
The adsorption capacity at different flow-rates are
given in Figure 1. The adsorption capacity decreased significantly from 17.2
mg/g to 6.9 mg/g polymer with the increase of the flow-rate from 0.5 ml/min to
3.0 ml/min. One of the explanation for such phenomenon would be a faster lig-and-metal
ion (i.e., alizarin yellow) dissociation rate compared to the association
rate. Hence, the adsorbate (i.e., Al(III) ions) would pass through the
magnetically stabilized column without adsorption at high flow-rate. Second
explanation could be that the increased nonideal flow hydrodynamics of liquid
phase and the solid phase for magnetically stabilized fluidized bed. These
phenomena can be summarized by the increase of the axial dispersion
coefficient in the axial dispersion model [25].
Adsorption capacity
Figure 2 shows the adsorption profile of Al(III) ions.
The amount of Al(III) ions adsorbed per unit mass of the polymer (i.e.
adsorption capacity) increased first with the initial concentration of Al(III)
ions then reached a plateau value at about an initial Al(III) ions
concentrations of 50 mg/L, which represents saturation of the active attachment
sites (which are available for Al(III) ions) on the beads. The maximum
adsorption capacity of Al(III) ions was of 647 |imol/g (17.5 mg/g). Unit mass
of the mPHEMA beads carries 208 цто1 alizarin yellow which was found by
elemental analysis. From the mass-stoichi-ometry, it seems that one attached
alizarin yellow molecule interacts with around three Al(III) ions. Since
alizarin yellow has seven coordinating sites of a single sulphur and six oxygen
atoms, it can form a ternary complex which is coordinated with water molecules
at vacant coordination sites of metal-alizarin yellow complexes.
It should be noted that the nonspecific adsorption
(adsorption on plain mPHEMA beads) of Al(III) ions was relatively low (0.63
mg/g). mPHEMA beads do not contain ion exchange or chelating groups. Preferred
coordination structure and preferred coordinating ligand atom may be utilized
for this adsorption. Al(III) ions may interact with Oxygen atoms as the
ligand. Diffusion of Al(III) ions into the swollen polymeric structure and
retention in the pores may also contribute to this nonspecific Al(III)
adsorption.
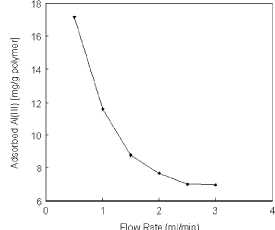
Figure 1
Effect of flow-rate on Al(III) adsorption.
Alizarin yellow loading: 208 |imol/g; Al(III) concentration: 50 mg/L; pH: 5.0;
T: 25°C.
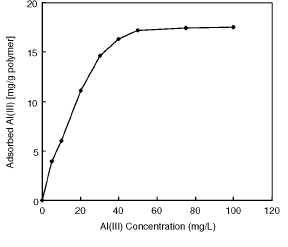
Figure 2
Effects of Al(III) concentration on Al(III)
adsorption. Alizarin yellow loading: 208 |imol/g; Flow-rate: 0.5 ml/min; pH:
5.0; Adsorption time: 60 min; T: 25°C.
Adsorption isotherms
An adsorption isotherm is used to characterize the
interactions of each molecule with the adsorbent. In this case it provides a
relationship between the concentration of the Al(III) ions in the solution and
the amount of Al(III) ions adsorbed on the solid phase when the two phases are
at equilibrium. The Langmuir adsorption model assumes that the species are
adsorbed at a fixed number of well-defined sites, each of which is capable of
holding only one molecule. These sites are also assumed to be energetically
equivalent, and distant from each other so that there are no interactions
between molecules adsorbed on adjacent sites.
Adsorption isotherms were used to evaluate adsorption
properties. The Langmuir adsorption isotherm is expressed by Equation 1. The
corresponding transformations of the equilibrium data for Al(III) gave rise to
a linear plot, indicating that the Langmuir model could be applied in these
systems and described by the equation:
![]()
where Q is the adsorbed amount of Al(III) (mg/g), Ceqis
the equilibrium Al(III) concentration (mg/mL), b is the Langmuir constant
(mL/mg) and, Qmax is the maximum adsorption capacity (mg/g). This
equation can be linearized so that
![]()
The plot of Ceq versus Ceq/Q was
employed to generate the intercept of 1/Qmax.b and the slope of 1/Qmax.
The maximum adsorption capacity (Qmax) data
for the adsorption of Al(III) was obtained from the experimental data. The
correlation coefficient (R2) was 0.989. The Langmuir adsorption
model can be applied in this affinity adsorbent system. Maximum adsorption
capacities determined using Langmuir isotherms show that dynamic capacity
(25.3 mg/g) was somewhat higher than the batch capacity (12.6 mg/g). The
dissociation constants for Al(III) were determined using the Langmuir isotherm
equation to be 27.3 mM (MSFB) and 6.7 mM (batch system), indicating medium
affinity, which was typical for pseudospecific affinity ligands.
Effect of pH
Metal ion adsorption onto specific adsorbents is pH
dependent. In the absence of complexing agents, the hydrolysis and
precipitation of the metal ions are affected by the concentration and form of
soluble metal species. The solubility of metal ions is governed by hydroxide or
carbonate concentration. Hydrolysis of metal ions becomes significant at
approximately pH 7.5-8.5. Therefore, in the present study, we changed the pH
range between 2.0-7.0. The effect of pH on the Al(III) adsorption of this
alizarin yellow-attached mPHEMA beads is also shown in Figure 3. The magnetic
mPHEMA beads exhibited a low affinity in acidic condition (pH < 4.0), a
somewhat higher affinity between pH 4.0 and 7.0. High adsorption capacities at
around neutral pH values imply that Al(III) ions interact with dye molecules
not only through the oxygen atoms by chelating, but also electrostatically
through sulfonate groups, which are ionized at neutral pH.
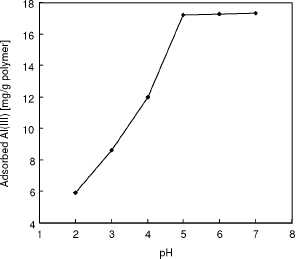
Figure 3
Effects of pH on Al(III) adsorption. Alizarin yellow loading: 208 u,mol/g; Flow-rate: 0.5 ml/min; Al(III) concentration: 50 mg/L; Adsorption time: 60 min; T: 25°C.
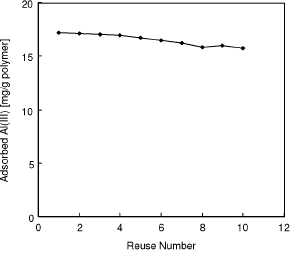
Figure 4
Repeated use of dye-attached mPHEMA beads.
Alizarin yellow loading: 208 u,mol/g; Flow-rate: 0.5 ml/min; Al(III) concentration:
50 mg/L; Adsorption time: 60 min; pH: 5.0; T: 25°C.
Competitive adsorption
Competitive adsorption of the metal ions from tap
water in Ankara and dialysis water (reverse osmosis) was also investigated. The
water containing different amounts of each metal ion was treated with dye beads
in MSFB. Table 1 and 2 show the adsorbed amounts for each metal ion. The
adsorption capacity of the dye-attached mPHEMA beads for Cu(II) and Al(III)
ions was higher than that for other ions. But it should also be noted that the
extent of adsorption of each type of metal ion is strongly dependent upon their
relative concentrations within the medium.
The World Health Organization (WHO) and the European
Community (EC) guide values for Al(III) ions for tap water is 200 ng/ml
[26,27]. Al(III) concentrations both in tap water and dialysis water are below
this value. It should be noted that polymer treatment (i.e, adsorption) significantly
decreases the metal content and these purified waters can be used safely
especially for the preparation of dialysis solutions. Magnetic dye-affinity
beads exhibits the following metal ion affinity sequence: Al(III) > Cu(II)
> Fe(III) > Zn(II).
Desorption and repeated
use
Desorption ratios were very high (up to 97.6%) with
the eluant system and under conditions used. When HNO3 is used as a
desorption agent, the coordination spheres of chelated Al(III) ions are
disrupted and subsequently Al(III) ions are released from the solid surface
into the desorption medium. Therefore, we conclude that HNO3 is a
suitable desorption agent for the dye adsorbents, and allows their repeated
use. In order to show the reusability of the dye-attached mPHEMA beads,
adsorption-desorp-tion cycle was repeated ten times by using the same sample
of affinity adsorbent. As can be seen from Figure 4, adsorption capacities did
not noticeable change during the repeated adsorption-desorption cycles.
Comparison of magnetically stabilized fluidized bed
and batch system
As can be seen in Figure 5, maximum Al(III) adsorption
from aqueous solution is 11.8 mg/g for batch system and 17.5 mg/g for MSFB
system. These results indicated that the adsorption capacity obtained in MSFB
system is considerably higher than obtained in batch sytstem. This means, in
equilibrium binding experiments, maximum capacity was 38.8% lower as compared
to the value obtained in MSFB. This result could be explained in two ways. (i)
The dye ligand-Al(III) dissociation rate in the batch system is higher than the
association rate in the MSFB system. (ii) Alizarin yellow ligand is found both
on
|
Table 1: Aluminium removal from the
tap water. |
||||||
|
Metal Ion % |
Concentration of Metal Ions (ng/ml) |
Metal Ion Adsorption (|xg/g) |
Adsorbed Metal Ions (%) |
|||
|
Al(III) |
80.1 |
40.3 ±0.1 |
98.9 |
|||
|
Fe(III) |
32.1 |
10.8 ± 0.2 |
54.3 |
|||
|
Cu(II) |
145.3 |
28.3 ± 0.2 |
35.1 |
|||
|
Cd(II) |
0.05 |
nd |
- |
|||
|
Pb(II) |
0.03 |
nd |
- |
|||
|
Zn(II) |
20.4 |
1.5 ±0.1 |
28.6 |
|||
|
Adsorption Conditions: Flow-rate: 0.5
ml/min; pH: 5.0; T: 25°C, nd: not determined. Each experiment was repeated
three times. |
||||||
|
Table 2: Aluminium removal from
dialysis water. |
||||||
|
Metal Ion % |
Concentration of Metal Ions (ng/ml) |
Metal Ion Adsorption (|xg/g) |
Adsorbed Metal Ions (%) |
|||
|
Al(III) |
18.96 |
9.46 ± 0.1 |
99.7 |
|||
|
Fe(III) |
0.05 |
- |
- |
|||
|
Cu(II) |
0.82 |
0.16 ±0.01 |
40.0 |
|||
|
Zn(II) |
1.26 |
0.44 ±0.01 |
69.8 |
|||
|
Adsorption Conditions: Flow-rate: 0.5
ml/min; pH: 5.0; T: 25°C. Each experiment was repeated three times. |
||||||
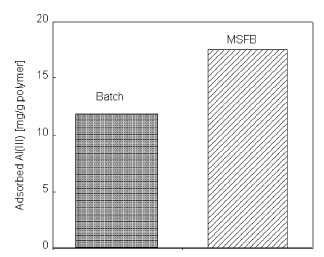
Figure 5
Comparison of MSFB and batch system.
Alizarin yellow loading: 208 |imol/g; Flow-rate: 0.5 ml/min; Al(III)
concentration: 50 mg/L; Adsorption time: 60 min; pH: 5.0; T: 25°C.
the surface and in the
pores of the magnetic beads. In the presence of flow, the Al(III) solution is
forced from the surface into the pores thus eliminating the surface diffusion.
Conclusions
The medical relevance of aluminium has stimulated the
development of cost and time effective separation techniques including
polymeric carriers. Magnetic adsorbents have several potential advantages over
conventional adsorbents [28-32]. The magnetically stabilized columns require
faster processing times and high flow-rates with a much lower operating
pressure than a packed bed column. In this study, mPHEMA beads, in the size
fraction of 80-120 |im, were produced by a dispersion polymerization of EGDMA
and HEMA in the presence of magnetite particles. These novel magnetic beads
were then successfully attached with reactive dye-ligand, namely alizarin
yellow. The highest dye loading was 208 |imol/g. Al(III) adsorption capacity of
the beads decreased with an increase in the flow-rate. The maximum Al(III)
adsorption was observed at pH 4.0. Al(III) adsorption onto the mPHEMA beads was
negligible (0.63 mg/g). Higher adsorption values (up to 17.5 mg/g) were
observed using alizarin yellow attached mPHEMA beads for the adsorption of
Al(III) ions from aqueous solutions. Al(III) ions could be repeatedly adsorbed
and desorbed without significant losses in their adsorption capacities.
References
- Martin
RB: The chemistry of aluminum as related to biology and medicine. Clin
Chem 1986, 32:1797-1806.
- Wills MR, Savory J: Aluminium poisoning:
dialysis encephalopathy. Lancet 1983, ii:29-34.
- Martyn
CN, Barker DJP, Osmond C, Harris EC, Edwardson JA, Lacey RF: Geographical
relation between Alzheimer's disease and aluminium in drinking water.
Lancet 1989, i:59-62.
- Nicolini
M, Zatta PF, Carain B, Eds: Aluminum in Chemistry, Biology and Medicine.
Cortina International, Verona 1991.
- Plessi
M, Monzani A: Aluminium determination in bottled mineral waters by
electro-thermal
atomic absorption
spectrometry. J Food Compos Anal 1995, 8:21 -26.
- Chang
TMS, Barre P: Effect of desferrioxamine on removal of aluminium and iron
by coated charcoal haemoperfusion and haemodialysis. Lancet 1983, ii:1051.
- Ackrill
P, Ralston AJ, Day JP, Hodge KC: Successful removal of aluminium from
patient with dialysis encephalopathy. Lancet 1980, ii:692-693.
- Altmann
P, Plowman , Marsh F, Cunningham J: Aluminium chela-tion therapy in dialysis
patients: evidence for inhibition of haemoglobin synthesis by low levels
of aluminium. Lancet ii:1012-1014.
- Gerhardsson L, Oskarsson A, Skerfving S: Acid
precipitation-effects on trace elements and human health. Sci Total
Environ 1994, 153:237-245.
- Denizli A,
Salih B, Kavakli C, Piskin E:
Dye-incorporated poly(EGDMA-HEMA) micro-spheres as specific
sorbents for aluminium removal. J Chromatogr B 1997, 698:89-96.
- Say
R, Denizli A: Preparation of magnetic dye affinity adsorbent and its use
in the removal of aluminium ions. J Biomater Sci PolymEd 2001,
12:1059-1073.
- Xue
B, Sun Y: Protein adsorption equilibria and kinetics to a poly(vinyl
alcohol)-based magnetic affinity support. J Chromatogr A 2001, 921:109-1
19.
- Burns
MA, Kvesitadze GI, Graves DJ: Dried calcium alginate/ magnetite spheres: a
new support for chromatographic separations and enzyme immobilization.
Biotechnol Bioeng 1985, 27:137-145.
- Bilkova
Z, Slovakova M, LyckaA, Horak D, Lenfeld J, Turkova J, Chu-racekJ:
Oriented immobilization of galactose oxidase to bead and magnetic bead
cellulose and poly(HEMA-co-EDMA) and magnetic poly(HEMA-co-EDMA)
microspheres. J Chromatogr В 2002, 770:25-34.
- O'Brien
SM, Thomas ORT, Dunnill P: Nonporous magnetic che-lator supports for
protein recovery by immobilised metal affinity adsorption. J Biotechnol
1996, 50:1 3-25.
- Rad
AY, Yavuz H, Denizli A: Bilirubin removal from human plasma with albumin
immobilised magnetic poly(2-Hydrox-yethyl methacrylate) beads. Macromol
Biosci 2003, 3:471-476.
- Martin
C, CuellarJ: Synthesis of a novel magnetic resin and the study of
equilibrium in cation exchange with amino acids. Ind Eng Chem Res 2004,
43:475-485.
- Tong
XD, Xue B, Sun Y: A novel magnetic affinity support for protein adsorption and
purification. Biotechnol Prog
2001, 17:1 34-1 39.
- Cocker
TM, Fee CJ, Evans RA: Preparation of magnetically susceptible
polyacrylamide/magnetite beads for use in magnetically stabilized fluidized bed chromatography. Biotechnol Bioeng 1997,53:79-87.
- Chetty
AS, Gabis DH, Burns MA: Overcoming support limitations in magnetically
stabilized fluidized bed separations. Powder Technol 1991, 64:165-174.
- Arica
Y, Yavuz H, Patir S, Denizli A: Immobilization of glucoamy-lase onto
spacer-arm attached magnetic poly(methylmeth-acrylate) microspheres:
characterization and application to a continuous flow reactor. J Mol Catal
B 2000, 1 1:127-138.
- Akgol
S, Denizli A: Novel metal-chelate affinity sorbents for reversible use
in catalase adsorption. J
Mol Catal B 2004, 28:7-14.
- Odabasi M,
Denizli A:
Polyhydroxyethylmethacrylate
based magnetic
DNA-affinity beads for
anti-DNA antibody removal
from systemic lupus erythematosus patient plasma. J Chromatogr B 2001,
760:137-148.
- Denizli
A, Say R, Piskin E: Removal of aluminium by alizarin yellow attached
magnetic poly(2-hydroxyethyl methacrylate) beads. React Funct Polym 2003,
55:99-107.
- Tong
XD, Sun Y: Application of magnetic agarose support in liquid magnetically stabilized
fluidized bed for
protein adsorption. Biotechnol Prog 2003, 19:1721 -1727.
- EEC-European Economic Community
Resolution 80/177. Official
J Eur Comm Directives CEE 80/777 1980.
- WHO,
Directive de qualite pour l'eau de boisson. Criteres d'hygiene et
documentation a l'appui, Geneve 1986, 2:254.
- Odabasi
M, Denizli A: Cibacron Blue F3GA-attached magnetic poly(2-hydroxyethyl
methacrylate) beads for human serum albumin adsorption. Polym Int 2004,
53:332-338.
- Odabasi
M, Denizli A: Cibacron Blue F3GA incorporated magnetic
poly(2-hydroxyethyl methacrylate) beads for lysozyme adsorption.J Appl
Polym Sci 2004, 93:719-725.
- Odabasi
M, Uzun L, Denizli A: Porous magnetic chelator support for albumin adsorption by immobilized metal affinity
separation. J Appl Polym Sci 2004, 93:2501 -2510.
- Akgol
S, Turkmen D, Denizli A: Cu(II)-incorporated, histidine-containing, magnetic-metal-complexing beads
as specific sorbents for
the metal chelate affinity of albumin.J Appl Polym Sci 2004, 93:2669-2677.
- Ozkara
S, Akgol S, Canak Y, Denizli A: A novel magnetic adsorbent for
immunoglobulin-G purification in magnetically stabilized fluidized bed.
Biotechnol Prog 2004 in press.