Magnetic alginate microparticles for purification of a-amylases
M. Safafikovaa, I. Royb, M.N.
Guptab, I. Safafikc
1 Laboratory of
Biochemistry and Microbiology, Institute of Landscape Ecology, Na Sadkach 7, 3
70 05 Ceske Budejovice, Czech Republic
b Chemistry
Department, Indian Institute of Technology, Delhi, Hauz Khas, New Delhi 110016,
India
c Department of
General Biology, University of South Bohemia, Branisovska 31, 370 05 Ceske
Budejovice, Czech Republic
Abstract
Spherical magnetic alginate microparticles (25-60 fim
in diameter) were prepared using the microemulsion system, with water-saturated
1-pentanol as the organic phase. The limited solubility of 1-pentanol in water
enabled simple removal of the organic solvent from the prepared beads with water
solution. The prepared alginate microparticles were used as magnetic affinity
adsorbents for specific purification of a-amylases. Enzyme activity was eluted by 1.0 M
maltose. a-Amylases from Bacillus
amy-loliquefaciens and porcine pancreatic acetone powder were purified 9- and
12-fold with 88 and 96% activity recovery, respectively.
Keywords: Alginate;
Ferrofluid; Magnetic fluid; Magnetic microparticles; Magnetic nanoparticles;
Amylases
1. Introduction
Alginates (polysaccharides consisting of man-nuronic and guluronic acids) are naturally occurring biopolymers and have many applications in various areas of biosciences and biotechnology (e.g. as a matrix for the entrapment and/or delivery of a variety of proteins and cells), and in the food and beverage industry (as a thickening or a gelling agent and a colloidal stabilizer; Smidsrod and Skjåk-Bræk, 1990).
Alginates offer a relatively inert aqueous environment
within the matrix and high gel porosity allows high diffusion rates of
macromolecules. Alginates also show unexpected affinity towards a-amylases (Roy et al., 2000).
For biotechnology applications, alginate is mainly used in the bead form
usually prepared by a syringe with a needle; the diameter and shape of the
beads formed is dependent on the size of the needle and the viscosity of the
alginate solution (Smidsrod and Skjåk-Bræk, 1990).
Several well known methods (atomization, spraying and
water-in-oil emulsifica-tion methods) can also be used to prepare alginate
microbeads that are less than 0.2 mm in diameter (Gombotz and Wee, 1998).
Alginate beads with incorporated
ferromagnetic materials represent very promising materials for various
applications. Magnetically modified beads easily manipulated with an external
magnetic field can be used when working with difficult to handle samples, such
as biological crude broths, which are often viscous suspensions (Safarik and
Safarikova, 1997; Safarikova and Safarik, 2001). Magnetic alginate beads have
been already used for affinity separation of various starch-degrading enzymes
such as a-amylase, (3-amylase, glucoamylase and
pullulanase (Teotia and Gupta, 2001, 2002). Other applications involve immobilization
of yeast cells (Brady et al., 1996) and immobilization of affinity ligands
(Burns et al., 1985). The goal of our work was to develop a simple procedure
for rapid preparation of magnetic alginate microparticles using microemulsion
procedure in a water-in-oil emulsion system. The microemulsion was formed using
water-saturated 1-pentanol as the oil phase. It is further shown that these
microparticles also constitute effective affinity materials for a-amylases. The
efficiency of the alginate microparticles was compared with alginate
macroparticles described in an earlier work (Teotia and Gupta, 2001). Alginate
microparticles were also used for the isolation of porcine pancreatic
a-amylase from porcine pancreatic acetone powder containing a number of
contaminating hydrolases. Thus, the selectivity of the alginate as a
macroaffinity ligand was established.
2. Materials and methods
2.1. Materials
Sodium alginate was from BDH Laboratory Supplies,
United Kingdom. 1-Pentanol was from Aldrich, USA while sodium dodecylsulphate
(SDS) was from Serva, Germany. Citrate-based magnetic fluid was prepared as
described recently (Domingo et al., 2001); the concentration of magnetite was
37 mg/ml (determined using a spectrophotometric procedure; Kiwada et al.,
1986). Porcine pancreatic acetone powder (sold as pancreatin) was purchased
from Sisco Research Laboratories, Mumbai, India. BAN 240 L (commercial
preparation of a-amylase from Bacillus amyloliquefa-ciens) was a product of
Novo Nordisk A/S, Denmark and was obtained from Arun & Co., Mumbai, India.
All other chemicals used were of analytical grade.
2.2. Preparation of magnetic alginate microparticles
Two milliliters of 2% sodium alginate solution was
added to 60 mg of SDS in a test tube. After thorough mixing and SDS
dissolution, 600 fxl of citrate stabilized ferrofluid was added and the content
was mixed well again. Eight milliliters of water-saturated 1-pentanol was then
added and the whole content
was vortexed using a vortex mixer (IKA minishaker,
Model MS1, obtained from Fisher Scientific, Czech Republic) at the maximum
speed for ca. 5 min. Then the content of the test tube was rapidly poured to
another vortexed test tube containing 10 ml of 5% calcium chloride solution and
vortexing continued for another 2 min. Then the bead suspension was allowed to
stand for 15 min, the beads were separated using an appropriate magnetic
separator and washed repeatedly with 5% calcium chloride solution until the
1-pentanol was washed out. Finally, the suspension was filtered through a sieve
(mesh size: 100 mm). The beads were stored in 5%
calcium chloride solution at 4 °C. The size of magnetic alginate microparticles
was measured using optical microscopy.
2.3. Estimation of enzyme activities and amount of
protein
Activity of a-amylases was estimated using starch as
the substrate (Dekker, 1977). In the case of porcine pancreatic amylase, the
assay was carried out at pH 6.9 (Dekker, 1977), whereas in the case of the
bacterial enzyme, the assay was done at pH 5.6 [Product sheet on BAN, NOVO
Nordisk A/S (1990)]. Bradford assay was used for the protein determination with
bovine serum albumin as a standard (Bradford, 1976).
2.4. Binding capacity of magnetic alginate
microparticles for a-amylases
Binding capacities of the microparticles were measured
by equilibrating 1 ml of different concentrations of the enzyme (bacterial
enzyme, 0.5-1 ml; porcine pancreatic enzyme, 0.4-1 ml, appropriately diluted in
the respective assay buffers up to a total volume of 1 ml) with 1 ml of the
magnetic alginate microparticles (settled volume, after decanting the excess
buffer) overnight at 25 °C. After equilibration, aliquots were removed and the
amylase activity in the supernatant was measured to calculate bound amylase
activity per ml of the alginate microparticles.
2.5. Purification of a-amylases
The magnetic alginate microparticles (16 ml, settled
volume) were equilibrated with 20 ml each of porcine pancreatic and bacterial
a-amylase preparations at
25 °C for 1 h. After equilibration, the microparticles
were washed with the respective assay buffers till the enzyme activity in the
washings dropped to zero. The microparticles were then equilibrated with 15 ml
of 1M maltose at 4 °C for 4 h. The alginate microparticles were then
magnetically separated and the eluate was dialyzed extensively against the
respective assay buffers (24 h at 4°C). The eluate was then checked for enzyme
activity.
3. Results
Microemulsion procedure with the organic solvent
partially miscible with water (1-pentanol) as the organic phase made it
possible to prepare spherical magnetic alginate microparticles having the
diameters in the range 25-60 (xm. The diameter of the prepared microparticles
varied slightly with the time of vortexing the emulsion. The magnetic phase was
homogeneously distributed throughout the particle volume (see Fig. 1). The
character of the organic phase (1-pentanol) enabled simple washing of the
prepared microparticles with calcium chloride solution which is a very cheap
and safe procedure. The magnetic alginate microparticles are easily
dispersible in water based solutions. The alginate magnetic microparticles also
bind bacterial and porcine pancreatic a-amylase activity just like the bigger
magnetic alginate particles (Teotiaand Gupta, 2001). The main advantage of
using smaller-sized particles is expected to be higher binding capacity. In
earlier work with alginate macroparticles, the binding capacity of 4.7 U
ml"1 (settled volume of magnetic particles) had been reported
with a commercial preparation of B. amyloliquefaciens a-amylase (BAN 240 L).
The alginate microparticles showed the binding capacity ca. seven times higher,
i.e. 36 U ml-1 (Fig. 2). Similar high capacity for porcine
pancreatic a-amylase (38Uml-1) was also observed (Fig. 3).
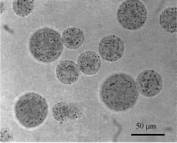
Fig. 1.
Optical microscopy of magnetic alginate microparticles (magnification: 400 x).
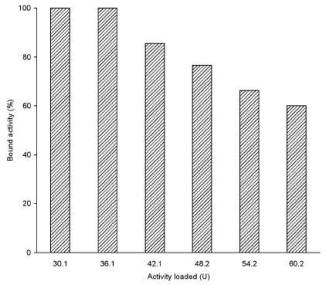
Fig. 2. Binding capacity of bacterial a-amylase on
magnetic alginate microparticles. Binding capacity was measured by
equilibrating 1 ml of different concentrations of the enzyme (0.5-1 ml of the
original enzyme solution diluted up to a total volume of 1 ml) with 1 ml of the
settled magnetic alginate beads overnight at 25 °C.
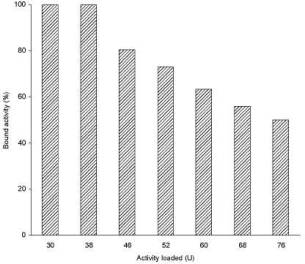
Fig. 3. Binding capacity of porcine pancreatic
a-amylase on magnetic alginate microparticles. Binding capacity was measured by
equilibrating 1 ml of different concentrations of the enzyme (0.4-1 ml of the
original enzyme solution diluted up to a total volume of 1 ml) with 1 ml of the
settled magnetic alginate beads overnight at 25 °C.
Table 1
Summary of the purification of a-amylase from BAN 240
L (a bacterial source) using magnetic alginate microparticles
|
Steps |
Activity (U) |
Protein (mg) |
Activity yield (%) |
Specific activity (Umg ') |
Purification factor |
|
Crude Wash Eluate (1 M maltose) |
580 1.5 511 |
8.3 6.8 0.8 |
100 0.3 88 |
70 0.2 638.7 |
1 - 9 |
The settled magnetic alginate microparticles (16 ml)
were equilibrated with 20 ml a-amylase at 25 °C for 1 h, washed with the assay
buffer and then equilibrated with 15 ml of 1 M maltose at 4°C for 4h. After
magnetic separation the eluate was dialyzed against the assay buffer (24 h at
4°C) and checked for enzyme activity.
Table 2
Summary of the purification of a-amylase from porcine
pancreatic extract using magnetic alginate microparticles
|
Steps |
Activity (U) |
Protein (mg) |
Activity yield (%) |
Specific activity (Umg ') |
Purification factor |
|
Crude Wash Eluate (1 M maltose) |
578.8 0.0 557.5 |
2.5 2.1 0.2 |
100 0 96 |
231.5 - 2787.5 |
1 - 12 |
The settled magnetic alginate microparticles (16ml)
were equilibrated with 20ml a-amylase at 25 °C for 1h, washed with the assay
buffer and then equilibrated with 15 ml of 1 M maltose at 4°C for 4h. After
magnetic separation the eluate was dialyzed against the assay buffer (24 h at
4°C) and checked for enzyme activity.
The magnetic microparticles could be used to purify
a-amylases from both these sources. It has been earlier shown that maltose
specifically elutes a-amylase from alginate beads (Sardar and Gupta, 1998).
Table 1 summarizes the purification process for BAN 240 L. The observed 88%
recovery of enzyme activity with 9-fold purification using alginate magnetic
microparticles is much better than 72% recovery with 3.6-fold purification
reported earlier using magnetic alginate macroparticles (Teotia and Gupta,
2001). Table 2 shows the purification of a-amylase from crude porcine
pancreatic acetone powder extract when 96% enzyme activity could be recovered with
12-fold purification. The specific activities indicate that the isolated
enzymes are of high purity (Mondal et al., 2003).
4. Discussion
The modified emulsion procedure using organic solvents
partially miscible with water as the oil phase (e.g. 1-pentanol) enables to
prepare magnetic alginate microparticles in a very simple way, without the need
of harsh organic solvents to remove the organic phase from the prepared beads.
This is a very important simplification of the existing approach. Magnetic
material is homogenously dispersed through the beads in sufficient amount
enabling efficient magnetic separation on a standard magnetic separator.
Prepared alginate microparticles were used for the
purification of selected a-amylases. The new magnetic alginate microparticles constitute much improved
affinity media for purification of a-amylases than larger particles. While only
the purification from mammalian and bacterial sources has been described here,
it is possible that just like the earlier macroparticles, these will also be
useful for isolation of a-amylases from other sources. Also, alginate has been shown to
constitute a macroaffinity ligand for other starch-degrading enzymes (Teotia
and Gupta, 2002), pectinase (Gupta et al., 1993), lipase (Sharma and Gupta,
2001) and phospholipase D (Sharma et al., 2000). Thus, the magnetic alginate
microparticles described here promise to be a multipurpose affinity media for
numerous enzymes of biochemical and biotechnological importance.
Acknowledgements
The research is a part of ILE Research Intention No.
AV0Z6087904. The experimental work was supported by the Ministry of Education
of the Czech Republic (Grant Project No. OC 523.80) and Grant Agency of the
Czech Academy of Sciences (Project No. S6087204). The partial supports provided
by Council for Scientific and Industrial Research (CSIR) (Extramural Division
and Technology Mission on Oilseeds, Pulses and Maize) and Department of Science
and Technology, both Government of India organizations, are gratefully acknowledged.
References
- Bradford, M.M.,
1976. A rapid and sensitive method for the quantitation of microgram
quantities of protein utilizing the principle of protein-dye binding.
Anal. Biochem. 72, 248-254.
- Brady, D., Nigam,
P., Marchant, R., McHale, L., McHale, A.P., 1996. Ethanol production at 45
°C by Kluyveromyces marxianus IMB3 immobilized in magnetically responsive
alginate matrices. Biotechnol. Lett. 18, 1213-1216.
- Burns, M.A.,
Kvesitadze, G.I., Graves, D.J., 1985. Dried calcium alginate magnetite
spheres—a new support for chromatographic separations and enzyme
immobilization. Biotechnol. Bioeng. 27, 137-145.
- Dekker, L.A.,
1977. Worthington Enzyme Manual. Worthington Biochemical Corp., Freehold,
NJ, pp. 173-176.
- Domingo, J.C.,
Mercadal, M., Petriz, J., De Madariaga, M.A., 2001. Preparation of
PEG-grafted immunomagnetoliposomes entrapping citrate stabilized
magnetite particles and their application in CD34+
cell sorting. J. Microencapsul. 18, 41— 54.
- Gombotz, W.R.,
Wee, S., 1998. Protein release
from alginate matrices. Adv. Drug Deliv. Rev. 31, 267-285.
- Gupta, M.N.,
Dong, G., Mattiasson, B., 1993.
Purification of endo-polygalacturonase by affinity precipitation using
alginate. Biotechnol. Appl. Biochem. 18, 321-327.
- Kiwada, H., Sato,
J., Yamada, S., Kato, Y., 1986. Feasibility of magnetic liposomes as a
targeting device for drugs. Chem. Pharm. Bull. 34.
- Mondal, K.,
Sharma, A., Gupta, M.N., 2003. Macroaffinity ligand facilitated three
phase partitioning (MLFTPP) of a-amylases using a modified alginate. Biotechnol. Prog. 19, 493-494.
- Roy, I., Sardar,
M., Gupta, M.N., 2000. Exploiting unusual affinity of usual
polysaccharides for separation of enzymes on fluidized beds. Enzyme
Microb. Technol. 27, 53-65.
- Safafik, I.,
Safafikov4 M., 1997. Overview of magnetic separations used in biochemical
and biotechnological applications.
In: Hafeli, U., Schutt, W., Teller J.,
Zborowski, M. (Eds.), Scientific and Clinical
Applications of Magnetic Carriers.
- Plenum Press, New
York, pp. 323-340. Safafikova, M., Safafik, I., 2001. The application of
magnetic techniques in biosciences. Magn. Electr. Sep. 10, 223-252.
Sardar, M., Gupta, M.N., 1998.
Alginate beads as an affinity material for alpha amylases. Bioseparation
7, 159-165.
- Sharma, S.,
Gupta, M.N., 2001. Alginate as a macroaffinity ligand and an additive for
enhanced activity and thermostability of lipases. Biotechnol. Appl.
Biochem. 33, 161-165.
- Sharma, S.,
Sharma, A., Gupta, M.N., 2000. One step purification of peanut
phospholipase D by precipitation with alginate. Bioseparation 9, 93-98.
- Smidsrad, O., Skjåk-Bræk,
G., 1990. Alginate as immobilization matrix for cells. Trends Biotechnol.
8, 71-78. Teotia, S., Gupta, M.N., 2001. Purification of alpha-amylases
using magnetic alginate beads. Appl. Biochem. Biotechnol. 90, 211-220.
- Teotia, S.,
Gupta, M.N., 2002.
Magnetite-alginate
beads for purification of
some starch degrading enzymes. Mol. Biotechnol. 20, 231-237.