Morphological Study of Saccharomyces
Cerevisiae Cells Treated With Magnetic Fluid
R. B. Azevedo, L. P. Silva, A. P. С Lemos, S. N. Bao, Z. G. M. Lacava, I. Safarik, M.
Safarikova, and P. C. Morais, Member, IEEE
Abstract
This paper shows that Saccharomyces
cerevisiae efficiently interact with magnetite-based ionic magnetic fluid,
leading to the formation of magnetically labeled cells which could be easily
separated from the system using an appropriated field-gradient-based magnetic
separator. Scanning and transmission electron microscopies were used to
investigate the interaction of Saccharomyces cerevisiae cells with magnetite
nanoparticles. The high-resolution microscopy data suggested that particle incorporation
occurs via an active process. Further, the microscopy data shows that the
particles did not reach the cell cytoplasm, staying in the periplasmatic space.
Index
Terms—Magnetic fluid (MF), scanning electron microscopy (SEM), transmission
electron microscopy (ТЕМ), yeast cells.
I. Introduction
THE use
of magnetic materials to support the development of field-gradient magnetic
separation technologies has attracted a great deal of attention in recent
years [1]-[3]. Applications of such technology span from cell separation [1]
to removal of actinides from wastewater [2]. Field-gradient magnetic
separation (FGMS) technologies commonly use micron-and nano-sized magnetic
particles, which are coupled to the target species for latter removal using a
field-gradient-based magnetic device [3]. Magnetic fluids (MFs), once properly
engineered to couple to a target, are excellent candidates to support FGMS
cleanup technologies addressed to wastewater from the industry. Wastewater
containing processed textile dyes, for instance, is an increasing source of
environmental contamination. Studies onbiodegradation, environmental impact,
and health effects of colorant materials, however, reveal the complexity of
the subject, not only due to the structural variety of these compounds, but
also as a result of the complex composition of effluents which they
contaminate. Therefore, the experimental research addressed to the remediation
of the environmental effects has been based on several possible strategies.
One of them assesses the elucidation of the degradation processes of a limited
number of dyes by selected microorganisms [4]. Various strains of yeasts are
among the microorganizms used to promote removal and degradation of dyes from
wastewater [5]. In addition, very recent data showed that yeast cells {Saccharomyces
cerevisiae) efficiently interact with magnetic nanoparticles stabilized as
low-pH ionic magnetic fluid, leading to the formation of magnetically labeled
cells which could be easily separated from the system using an appropriated
magnetic separator [6]. However, in order to take full advantage of the formed
cell-nanoparticle complex in establishing new technologies one needs to
investigate more precisely the nanoparticle cell-site distribution. In this
paper, scanning and transmission electron microscopies were used to investigate
the interaction of Saccharomyces cerevisiae cells with magnetic nanoparticles
after incubation of the cells with a low-pH ionic MF sample.
II. Materials and Methods
Baker's
yeast {Saccharomyces cerevisiae cells) was incubated with low-pH (perchloric
acid stabilization) ionic magnetic fluid. The magnetite-based ionic MF sample
was prepared using the standard procedure [7]. The nanoparticle concentration
within the MF sample (32.0 mg/mL) is given as the magnetite content determined
by a colorimetric method [8]. Procedure used to label the yeast cells with
magnetic nanoparticles is described as follows.
Compressed
baker's yeast (2 g) was suspended in saline (6 mL), centrifuged, and
resuspended in a 6-mL 0.1-M acetate buffer (pH 4.6). After further
centrifugation, the sediment was resuspended in acetate buffer to obtain ca 33%
yeast suspension (v/v; yeast cells volume determined after sedimentation for
24 h at 1 g). Magnetic labeling of the yeast cells was performed using 3 mL of
the yeast suspension and 1 mL of the MF sample. The yeast suspension was mixed
with the MF and then incubated at room temperature for one hour without mixing.
After this time period the majority of yeast cells were magnetically modified
by the added MF sample (the cells responded to external magnetic field).
Nonmagnetic yeast cells and residual magnetic fluid were removed by repeated
static magnetic separation using acetate buffer (once) and saline as washing
liquids, respectively, until the supernatant was clear.
Alternatively,
cultured yeast cells were used for magnetic modification. Baker's yeast was
suspended in 1% unbuffered saccharose solution and cultivated at 30 °С for 2 h. The cultured cells were centrifuged and
treated essentially in the same way as compressed baker's yeast. Besides
labeling with MFs, heating of the yeast cells in boiling water for 15 min was
performed as well. The following treatment groups were considered: group
G1—cells were first cultured and then incubated with MF; group G2—cells were
first cultured, then incubated with MF, and finally heated; group
G3—noncultured cells were incubated with MF; group G4—noncultured cells were
first incubated with MF and then heated; group G5—cells were first cultured,
then heated, and finally incubated with MF; and group G6—noncultured cells were
first heated and then incubated with MF. After treatment, cells were harvested,
washed, fixed, and processed for scanning electron microscopy (SEM) analysis
and transmission electron microscopy (ТЕМ) analysis.
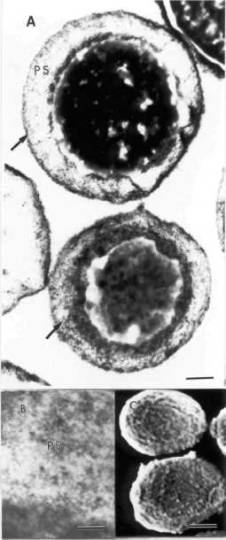
Fig. 1. ТЕМ pictures of G1-cells are shown in A and B. In A (arrows) and B, note
the presence of magnetic nanoparticles inside the PS. SEM micrograph of
G1-cells is shown in C. Note the bar sizes in A (0.4 цт), В (80 nm), and С (1 ftm).
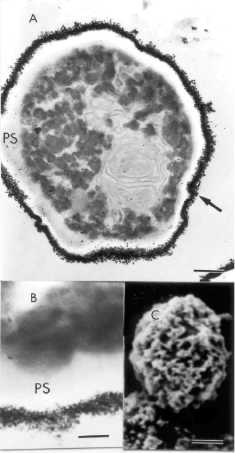
Fig. 2. ТЕМ pictures of G6-cells are shown in A and B. Note in A (arrow) and B,
that magnetic nanoparticles are outside the cells instead of in the
periplasmatic space (PS). SEM micrograph of G6-cells is shown in C. Very rough
surface is observed in С due to agglomeration
of magnetic nanoparticles (quite different from С in Fig. 1). Note the bar sizes in A (0.3 fim), В (80 nm), and С (0.7 fim).
III. Results and Discussion
In Fig.
1, SEM micrograph (C) showed smooth cell surface after G1 treatment. G1 and G2
treatments revealed similar results. For these two groups, ТЕМ pictures revealed small number of magnetic
nanoparticles on the cell surface and a huge amount of magnetic nanoparticles
inside the cells, as showed in Fig. 1 (A and B). Whereas the majority of nanoparticles
were observed between the cellular wall and plasmatic membrane, i.e.,
periplasmatic space (PS), few magnetic nanoparticles were found within the
cytoplasm.
In Fig.
2, SEM micrograph (C) showed rough cell surface after G6 treatment, due to agglomerates
of magnetic nanoparticles. G3-G6 treatments revealed similar results. In
addition, after G3-G6 treatments, the ТЕМ pictures showed very few magnetic nanoparticles inside the cells and a
large amount of them on the cell surface, as shown in Fig. 2 (A andB).
Regarding groups G3 and G4, where cells were not cultivated and no particles
were observed inside the cells, it is clear that the cultivation step is
fundamental forMF internalizationby the baker's yeast. This result is probably
related to the dormant process occurring in the commercial baker's yeast before
incubation. After incubation, the cells go into exponential phase of growth,
accelerating their cellular functions, including endocytosis capacity, which is
the potential phenomenon behind the incorporation of magnetic nanoparticles by
the yeast cells in our experiment. This hypothesis seems to be quite reasonable
once groups heated before incubation with MF (G5 and G6) did not internalize
magnetic particles. This is a strong indication that nanoparticle
internal-ization is an active instead of a passive phenomenon. Table I
summarizes the G1-G6 group treatments.
At this
point, it is interesting to observe that cells first cultivated and then
treated with MF, before heating (groups G1 and G2), were able to incorporate
nanoparticles in the periplasmatic space. Note that magnetic nanoparticles were
able to cross the cellular wall, but not the cellular membrane (Fig. 1A and B).
It is well known that cellular membrane is more selective than cellular wall,
thus supporting our findings. This observation does not interfere with the main
goal of the present study, i.e., labeling of yeast cells with magnetic
nanoparticles. In addition, neither incubation with MF nor heating after
incubation interfere in the baker's yeast capacity in adsorbing dyes. In fact,
it has been reported that the dye adsorption capacity is increased when yeast
cells are heated for 15 min [6]. Therefore, the order of heating and magnetic
modification of yeast cells gain importance from the point of view of cell
adsorption capacity.
IV. Conclusion
In
conclusion, baker's yeast incorporation of magnetic nanoparticles, stabilized
as ionic magnetic fluids, is an active process that needs to be performed with
cultivated Saccha-romyces cerevisiae cells. We have observed a significant
nanoparticle cell uptake which does not interfere with the dye adsorption
capacity of the yeast cells. Therefore, the approach presented in this paper to
magnetically load yeast cells represents a way to produce a promising material
for environmental bioremediation technologies.
References
- I. Safarik and M. Safarikova,
"Use of magnetic techniques for the isolation of cells," J.
Chromatogr. B, vol. 722, pp. 33-53, 1999.
- A. D. Ebner, J. A. Ritter, H.
J. Ploehn, R. L. Kochen, and J. D. Navratil, "New magnetic
field-enhanced process for the treatment of aqueous wastes," Separ. Sci.
Technol, vol. 34, pp. 1277-1300, 1999.
- S. Kurinobu, J. Uesugi, Y.
Utumi, and H. Kasahara, "Performance of HGMS filter and recycling of
magnetic seeding material on magnetic seeding method," IEEE Trans.
Magn., vol. 35, pp. 4067^069, Sept. 1999.
- T. Robinson, G. McMullan, R.
Marchant, and P. Nigam, "Remediation of dyes in textile effluent: A
critical review on current treatment technologies with a proposed
alternative," Bioresource Technol., vol. 77, pp. 247-255, 2001.
- M. A. M. Martins, M. H.
Cardoso, M. J. Queiroz, M. T. Ramalho, and A. M. O. Campos,
"Biodegradation of azo dyes by the yeast Candida zeylanoides in batch
aerated cultures," Chemosphere, vol. 38, pp. 2455-2460, 1999.
- I. Safarik, L. Ptackova, and M.
Safarikova, "Adsorption of dyes on magnetically labeled baker'syeast
cells, "Eur. Cells Mater,vol. 3,pp. 52-55, 2002.
- R. Massart, "Preparation
of aqueous magnetic liquids in alkaline and acidic media," IEEE
Trans. Magn., vol. MAG-17, pp. 1247-1248, Mar. 1981.
- H. Kiwada, J. Sato, and S.
Yamada, "Feasibility of magnetic liposomes as a targeting device for
drugs," Chem. Pharm. Bull, vol. 34, pp. 4253-4258, 1986.