Magnetic techniques for the isolation and
purification of proteins and peptides
Ivo Safarik*1,2 and Mirka
Safarikova1
Address:
1Laboratory of Biochemistry and
Microbiology, Institute of Landscape Ecology, Academy of Sciences, Na Sadkach
7, 370 05 Ceske Budejovice, Czech Republic and
2Department of General Biology, University
of South Bohemia, Branisovska 31, 370 05 Ceske Budejovice, Czech Republic
Email: Ivo Safarik* - ivosaf@yahoo.com; Mirka
Safarikova - mirkasaf@uek.cas.cz
* Corresponding author
Abstract
Isolation and separation of specific molecules is used
in almost all areas of biosciences and biotechnology. Diverse procedures can be
used to achieve this goal. Recently, increased attention has been paid to the
development and application of magnetic separation techniques, which employ
small magnetic particles. The purpose of this review paper is to summarize
various methodologies, strategies and materials which can be used for the
isolation and purification of target proteins and peptides with the help of
magnetic field. An extensive list of realised purification procedures documents
the efficiency of magnetic separation techniques.
Introduction
Isolation, separation and purification of various
types of proteins and peptides, as well as of other specific molecules, is
used in almost all branches of biosciences and biotechnologies. Separation
science and technology is thus very important area necessary for further
developments in bio-oriented research and technology. New separation
techniques, capable of treating dilute solutions or solutions containing only
minute amounts of target molecules in the presence of vast amounts of
accompanying compounds in both small and large-scale processes, even in the
presence of particulate matter, are necessary.
In the area of biosciences and biotechnology the
isolation of proteins and peptides is usually performed using variety of
chromatography, electrophoretic, ultrafiltration, precipitation and other
procedures, affinity chromatography being one of the most important
techniques. Affinity ligand techniques represent currently the most powerful
tool available to the downstream processing both in term of their selectivity
and recovery. The strength of column affinity chromatography has been shown in
thousands of successful applications, especially in the laboratory scale.
However, the disadvantage of all standard column liquid chromatography
procedures is the impossibility of the standard column systems to cope with the
samples containing particulate material so they are not suitable for work in
early stages of the isolation/purification process where suspended solid and
fouling components are present in the sample. In this case magnetic affinity,
ion-exchange, hydrophobic or adsorption batch separation processes, applications
of magnetically stabilized fluid-ized beds or magnetically modified two-phase
systems have shown their usefulness.
The basic principle of batch magnetic separation is
very simple. Magnetic carriers bearing an immobilized affinity or hydrophobic
ligand or ion-exchange groups, or magnetic biopolymer particles having
affinity to the isolated structure, are mixed with a sample containing target
compound(s). Samples may be crude cell lysates, whole
blood, plasma, ascites fluid, milk, whey, urine, cultivation media, wastes from
food and fermentation industry and many others. Following an incubation period
when the target compound(s) bind to the magnetic particles the whole magnetic
complex is easily and rapidly removed from the sample using an appropriate
magnetic separator. After washing out the contaminants, the isolated target
compound(s) can be eluted and used for further work.
Magnetic separation techniques have several advantages
in comparison with standard separation procedures. This process is usually very
simple, with only a few handling steps. All the steps of the purification
procedure can take place in one single test tube or another vessel. There is no
need for expensive liquid chromatography systems, centrifuges, filters or
other equipment. The separation process can be performed directly in crude
samples containing suspended solid material. In some cases (e.g., isolation of
intracellular proteins) it is even possible to integrate the disintegration and
separation steps and thus shorten the total separation time [1]. Due to the
magnetic properties of magnetic adsorbents (and diamagnetic properties of
majority of the contaminating molecules and particles present in the treated
sample), they can be relatively easily and selectively removed from the sample.
In fact, magnetic separation is the only feasible method for recovery of small
magnetic particles (diameter ca 0.1 - 1 цт) in the presence of biological debris and
other fouling material of similar size. Moreover, the power and efficiency of magnetic
separation procedures is especially useful at large-scale operations. The
magnetic separation techniques are also the basis of various automated
procedures, especially magnetic-particle based immunoassay systems for the
determination of a variety of analytes, among them proteins and peptides.
Several automated systems for the separation of proteins or nucleic acids have
become available recently.
Magnetic separation is usually very gentle to the
target proteins or peptides. Even large protein complexes that tend to be
broken up by traditional column chromatography techniques may remain intact
when using the very gentle magnetic separation procedure [2]. Both the reduced
shearing forces and the higher protein concentration throughout the isolation
process positively influence the separation process.
Separation of target proteins using standard
chromatography techniques often leads to the large volume of diluted protein
solution. In this case appropriate magnetic particles can be used for their concentration
instead of ultrafil-tration, precipitation etc. [3].
The purpose of this review is to summarize various
methodologies and strategies which can be employed for the isolation and
purification of target proteins and peptides with the help of magnetic
materials. An extensive list of realised purification procedures documents the
efficiency of magnetic separation techniques. All these information will help
the scientists to select the optimal magnetic material and the purification
procedure.
Necessary materials and
equipment
The basic equipment for laboratory experiments is very
simple. Magnetic carriers with immobilized affinity or hydrophobic ligands,
magnetic particles prepared from a biopolymer exhibiting affinity for the
target compound(s) or magnetic ion-exchangers are usually used to perform the
isolation procedure. Magnetic separators of different types can be used for
magnetic separations, but many times cheap strong permanent magnets are equally
efficient, especially in preliminary experiments.
Magnetic carriers and adsorbents can be either
prepared in the laboratory, or commercially available ones can be used. Such
carriers are usually available in the form of magnetic particles prepared from
various synthetic polymers, biopolymers or porous glass, or magnetic particles
based on the inorganic magnetic materials such as surface modified magnetite
can be used. Many of the particles behave like superparamagnetic ones
responding to an external magnetic field, but not interacting themselves in the
absence of magnetic field. This is important due to the fact that magnetic
particles can be easily resuspended and remain in suspension for a long time.
In most cases, the diameter of the particles differs from ca 50 nm to approx.
10 |im. However, also larger magnetic affinity particles, with the diameters up
to millimetre range, have been successfully used [4]. Magnetic particles
having the diameter larger than ca 1 |im can be easily separated using simple
magnetic separators, while separation of smaller particles (magnetic colloids
with the particle size ranging between tens and hundreds of nanometers) may
require the usage of high gradient magnetic separators.
Commercially available magnetic particles can be
obtained from a variety of companies. In most cases polystyrene is used as a
polymer matrix, but carriers based on cellulose, agarose, silica, porous glass
or silanized magnetic particles are also available. Examples of magnetic
particles used (or usable) for proteins and peptides separation can be found
elsewhere [5-7].
Particles with immobilised affinity ligands are
available for magnetic affinity adsorption. Streptavidin, antibodies, protein A
and Protein G are used most often in the course of protein and peptides
isolation. Magnetic particles with above mentioned immobilised ligands can also
serve as generic solid phases to which native or modified affinity ligands can
be immobilised (e.g., antibodies in the case of immobilised protein A, protein
G or secondary antibodies, biotinylated molecules in the case of immobilised
strep tavidin).
Also some other affinity ligands (e.g.,
nitrilotriacetic acid, glutathione, trypsin, trypsin inhibitor, gelatine etc.)
are already immobilised to commercially available carriers. To immobilise other
ligands of interest to both commercial and laboratory made magnetic particles
standard procedures used in affinity chromatography can be employed. Usually
functional groups available on the surface of magnetic particles such as
-COOH, -OH or -NH2 are used for immobilisation, in some cases
magnetic particles are available already in the activated form (e.g.,
tosy-lactivated, epoxyactivated etc).
In the laboratory magnetite (or similar magnetic
materials such as maghemite or ferrites) particles can be surface modified by
silanization. This process modifies the surface of the inorganic particles so
that appropriate functional groups become available, which enable easy
immobilisation of affinity ligands [8]. In exceptional cases enzyme activity
can be decreased as a result of usage of magnetic particles with exposed iron
oxides. In this case encapsulated microspheres, having an outer layer of pure
polymer, will be safer.
Biopolymers such as agarose, chitosan, kappa
carrageenan and alginate can be easily prepared in a magnetic form. In the
simplest way the biopolymer solution is mixed with magnetic particles and after
bulk gel formation the magnetic gel formed is mechanically broken into fine
particles [9]. Alternatively biopolymer solution containing dispersed magnetite
is dropped into a mixed hardening solution [4] or water-in-oil suspension
technique is used to prepare spherical particles [10].
Basically the same procedures can be used to prepare
magnetic particles from synthetic polymers such as polyacryla-mide,
poly(vinylalcohol) and many others [11].
In another approach used standard affinity or
ion-exchange chromatography material was post-magnetised by interaction of the
sorbent with water-based ferrofluid. Magnetic particles accumulated within the
pores of chromatography adsorbent thus modifying this material into magnetic
form [12,13]. Alternatively magnetic Sepharose or other agarose gels were
prepared by simple contact with freshly precipitated or finely powdered
magnetite [ 12,14].
Magnetoliposomes (magnetic derivatives of standard
liposomes), either in the original form or after immobilization of specific
proteins, have the potential for the separation of antiphospholipid antibodies
[15], IgG antibodies [16] and other proteins of interest [17].
Recently also non-spherical magnetic structures, such
as magnetic nanorods have been tested as possible adsorbent material for
specific separation of target proteins [18].
Magnetic separators are necessary to separate the magnetic
particles from the system. In the simplest approach, a small permanent magnet
can be used, but various magnetic separators employing strong rare-earth
magnets can be obtained at reasonable prices. Commercial laboratory scale batch
magnetic separators are usually made from magnets embedded in
disinfectant-proof material. The racks are constructed for separations in
Eppendorf micro-tubes, standard test tubes or centrifugation cuvettes, some of
them have a removable magnetic plate to facilitate easy washing of separated
magnetic particles. Other types of separators enable separations from the wells
of micro titra-tion plates and the flat magnetic separators are useful for
separation from larger volumes of suspensions (up to approx. 500 - 1000 ml).
Examples of typical batch magnetic separators are shown in Fig. 1.
Flow-through magnetic separators are usually more
expensive, and high gradient magnetic separators (HGMS) are the typical
examples. Laboratory scale HGMS is composed from a column packed with fine
magnetic grade stainless steel wool or small steel balls which is placed
between the poles of an appropriate magnet. The suspension is pumped through
the column, and magnetic particles are retained within the matrix. After
removal the column from the magnetic field, the particles are retrieved by flow
and usually by gentle vibration of the column.
For work in dense suspensions, open gradient magnetic
separators may be useful. A very simple experimental setup for the separation
of magnetic affinity adsorbents from litre volumes of suspensions was described
[19].
Currently many projects require the analysis of a high
number of individual proteins or variants. Therefore, methods are required that
allows multiparallel processing of different proteins. There are several
multiple systems for high throughput nucleic acid and proteins preparation
commercially available. The most often used approach for proteins isolation is
based on the isolation and assay of 6xHis-tagged recombinant proteins using
magnetic beads with Ni-nitriloacetic acid ligand [20]. The commercially
available platforms can be obtained from several companies such as Qiagen, USA
(BioRobot and BioSprint series), Tecan, Japan (Te-MagS) or Thermo Electron
Corporation, USA(KingFisher).
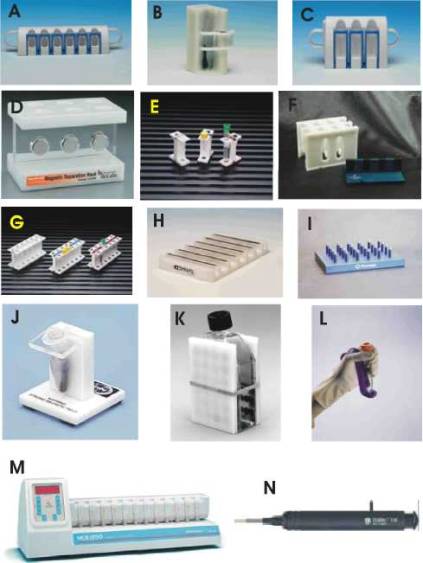
Figure 1
Examples of batch magnetic separators applicable
for magnetic separation of proteins and peptides. A: Dynal MPC -S for six
microtubes (Dynal, Norway); B: Dynal MPC - 1 for one test tube (Dynal, Norway);
C: Dynal MPC - L for six test tubes (Dynal, Norway); D: magnetic separator for
six Eppendorf tubes (New England BioLabs, USA); E: MagneSphere Technology
Magnetic Separation Stand, two position (Promega, USA); F: MagnaBot Large
Volume Magnetic Separation Device (Promega, USA); G: MagneSphere Technology
Magnetic Separation Stand, twelve-position (Promega, USA); H: Dynal MPC - 96 S
for 96-well microtitre plates (Dynal, Norway); I: MagnaBot 96 Magnetic
Separation Device for 96-well microtitre plates (Promega, USA); J: BioMag
Solo-Sep Microcentrifuge Tube Separator (Polysciences, USA); K: BioMag Flask
Separator (Polysciences, USA); L: Mag-neSil Magnetic Separation Unit (Promega,
USA); M: MCB 1200 processing system for 12 microtubes based on MixSep process
(Sigris Research, USA); N: PickPen magnetic tool (Bio-Nobile, Finland).
Reproduced with the permission of the above mentioned companies; the photos
were taken from their www pages.
Basic principles of
magnetic separations of proteins and peptides
Magnetic separations of proteins and peptides are
usually convenient and rapid. Nevertheless, several hints may be helpful to
obtain good results.
Proteins and peptides in the free form can be directly
isolated from different sources. Membrane bound proteins have to be usually
solubilized using appropriate detergents. When nuclei are broken during sample
preparation, DNA released into the lysate make the sample very viscous. This
DNA may be sheared by repeated passage up and down through a 21 gauge
hypodermic syringe needle before isolation of a target protein. Alternatively,
DNase can be added to enzymatically digest the DNA.
Magnetic beads in many cases exhibit low non-specific
binding of non-target molecules present in different samples. Certain samples
may still require preclearing to remove molecules which have high non-specific
binding activity. If preclearing is needed, the sample can be mixed with
magnetic beads not coated with the affinity ligand. In the case of
immunomagnetic separation, magnetic beads coated with secondary antibody or
with irrelevant antibodies have been used. The non-specific binding can also
be minimised by adding a non-ionic detergent both in the sample and in the
washing buffers after isolation of the target.
In general, magnetic affinity separations can be
performed in two different modes. In the direct method, an appropriate
affinity ligand is directly coupled to the magnetic particles or biopolymer
exhibiting the affinity towards target compound(s) is used in the course of
preparation of magnetic affinity particles. These particles are added to the
sample and target compounds then bind to them. In the indirect method the free
affinity ligand (in most cases an appropriate antibody) is added to the
solution or suspension to enable the interaction with the target compound. The
resulting complex is then captured by appropriate magnetic particles. In case
antibodies are used as free affinity ligands, magnetic particles with
immobilised secondary antibodies, protein A or protein G are used for
capturing of the complex. Alternatively the free affinity ligands can be
biotinylated and magnetic particles with immobilised streptavidin or avidin are
used to capture the complexes formed. In both methods, magnetic particles with
isolated target compound(s) are magnetically separated and then a series of
washing steps is performed to remove majority of contaminating compounds and
particles. The target compounds are then usually eluted, but for specific
applications (especially in molecular biology, bioanalytical chemistry or
environmental chemistry) they can be used still attached to the particles, such
as in the case of polymerase chain reaction, magnetic ELISA etc.
The two methods perform equally well, but, in general,
the direct technique is more controllable. The indirect procedure may perform
better if affinity ligands have poor affinity for the target compound.
In most cases, magnetic batch adsorption is used to
perform the separation step. This approach represents the simplest procedure
available, enabling to perform the whole separation in one test-tube or flask.
If larger magnetic particles (with diameters above ca 1 цш) are
used, simple magnetic separators can be employed. In case magnetic colloids
(diameters ranging between tens and hundreds of nanometres) are used as
affinity adsorbents, high-gradient magnetic separators have usually to be used
to remove the magnetic particles from the system.
Alternatively magnetically stabilised fluidised beds
(MSFB), which enable a continuous separation process, can be used. The use of
MSFB is an alternative to conventional column operation, such as packed-bed or
fluidised bed, especially for large-scale purification of biological products.
Magnetic stabilisation enables the expansion of a packed bed without mixing of
solid particles. High column efficiency, low pressure drop and elimination of
clogging can be reached [21,22].
Also non-magnetic chromatographic adsorbents can be
stabilized in magnetically stabilized fluidized beds if sufficient amount of
magnetically susceptible particles is also present. The minimum amount of
magnetic particles necessary to stabilize the bed is a function of various
parameters including the size and density of both particles, the magnetic
field strength, and the fluidization velocity. A variety of commercially
available affinity, ion-exchange, and adsorptive supports can be used in the
bed for continuous separations [23].
Biocompatible two phase systems, composed for example
from dextran and polyethylene glycol, are often used for isolation of
biologically active compounds, subcellular organelles and cells. One of the
disadvantages of this system is the slow separation of the phases when large
amounts of proteins and cellular components are present. The separation of the
phases can be accelerated by the addition of fine magnetic particles or
ferrofluids to the system followed by the application of a magnetic field. This
method seems to be useful when the two phases have very similar densities, the
volumetric ratio between the phases is very high or low, or the systems are
viscous. Magnetically enhanced phase separation usually increases the speed of
phase separation by a factor of about 10 in well-behaved systems, but it may
increase by a factor of many thousands in difficult systems. The addition of
ferrofluids and/or iron oxide particles was shown to have usually no influence
on enzyme partioning or enzyme activity [24,25].
Table 1: Examples of proteinases and peptidases purified
by magnetic techniques
|
Purified enzyme |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Aminopeptidase |
Arabidopsis |
Amine-terminated magnetic beads |
N-1 -Naphthylphthalamic acid |
KCl gradient elution |
[54] |
|
Angiotensin- converting enzyme |
Pig lung membranes |
Dynabeads |
Polyclonal antibodies |
|
[57] |
|
Bromelain |
Commercial preparation |
Polyacrylic acid - iron oxide magnetic
nanoparticles |
|
Elution with KCl solution |
[59] |
|
Caspase(recombinant, histidine-tailed) |
Human cells |
Magnetic agarose |
Ni-NTA |
Elution with SDS-PAGE buffer |
[60] |
|
Chymotrypsin |
Commercial preparation |
Magnetic chitosan beads |
|
Elution with N-acetyl-D- tryptophan |
[62] |
|
N Ia-protease(recombinant, histidine-
tagged) |
Plum Pox Virus |
Magnetic core and nickel- silica
composite matrix |
Ni2+ |
Elution with imidazole containing buffer |
[36] |
|
Proteinases |
Commercial sources |
Magnetic cross-linked erythrocytes |
|
Elution with low pH buffer |
[46] |
|
Proteinase, bacterial (Savinase) |
Bacillus clausii |
Silanized magnetite particles |
Bacitracin |
|
[84] |
|
Trypsin |
Porcine pancreatin |
Silanized magnetite particles |
p-Aminobenzamidine |
Elution with low pH buffer |
[50] |
|
|
Porcine pancreatin |
Magnetic polymer particles |
Soybean trypsin inhibitor |
Elution with low pH solution |
[86] |
|
|
Commercial preparation |
Silanized ferrite powder |
Soybean trypsin inhibitor |
|
[87] |
|
|
Commercial preparation |
Magnetic к-carrageenan particles |
Soybean trypsin inhibitor |
Elution with low pH solution, MSFB |
[88] [89] |
|
|
Commercial preparation |
Magnetic polyacrylamide beads |
Soybean trypsin inhibitor |
Magnetically stabilized fluidized beds |
[90] |
|
|
Commercial preparation |
Magnetic chitosan particles |
Aprotinin |
Elution with low pH solution |
[91] |
|
|
Commercial preparation |
Magnetic cross-linked erythrocytes |
|
Elution with low pH buffer; separation
from large volume sample |
[19] |
|
Urokinase |
Crude urokinase preparation |
Magnetic dextran, agarose, polyvinyl
alcohol, polyhydroxyethyl methacrylate microspheres |
p-Aminobenzamide, L- arginine methyl
ester, guanidine hexanoic acid or guanidine acetic acid |
|
[93] |
Proteins and peptides isolated using magnetic
techniques have to be usually eluted from the magnetic separation materials. In
most cases bound proteins and peptides can be submitted to standard elution
methods such as the change of pH, change of ionic strength, use of polarity
reducing agents (e.g., dioxane or ethyleneglycol) or the use of deforming
eluents containing chaotropic salts. Affinity elution (e.g., elution of
glycoproteins from lectin coated magnetic beads by the addition of free sugar)
may be both a very efficient and gentle procedure.
Examples of magnetic
separations of proteins and peptides
Magnetic affinity and ion-exchange separations have
been successfully used in various areas, such as molecular biology,
biochemistry, immunochemistry, enzymology, analytical chemistry, environmental
chemistry etc [26-29]. Tables 1, 2, 3, 4, 5, 6, 7, 8, 9 show some selected
applications of these techniques for proteins and peptides isolation.
In the case of proteins and peptides purifications, no
simple strategy for magnetic affinity separations exists. Various affinity
ligands have been immobilised on magnetic particles, or magnetic particles have
been prepared from biopolymers exhibiting the affinity for target enzymes or
lectins. Immunomagnetic particles, i.e. magnetic particles with immobilised
specific antibodies against the target structures, have been used for the
isolation of various antigens, both molecules and cells [5] and can thus be
used for the separation of specific proteins.
Table 2: Purification of lysozyme by magnetic techniques
|
Purified enzyme |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Lysozyme |
Hen egg white |
Magnetic chitin |
|
Elution with 0.01 M HCl |
[71] |
|
|
Hen egg white |
Magnetic acetylated chitosan |
|
Elution with 0.01 M HCl |
CT |
|
|
Commercial preparation |
Magnetic poly(2- hydroxyethyl
methacrylate) |
Cibacron Blue F3GA |
Elution with 1 M KSCN |
[72] |
|
|
|
Magnetic chitosan beads |
|
Magnetically stabilized fluidized bed |
[73] |
|
|
Ornithodoros moubata |
Magnetic chitin |
|
Elution with alkaline, high salt buffer |
[74] |
|
|
Commercial preparation |
Magnetic cross-linked polyvinyl alcohol |
Cibacron blue 3GA |
Elution with high salt buffer |
[52] |
|
|
|
Magnetite - polyacrylic acid
nanoparticles |
|
Ion-exchange separation |
[75] |
|
|
|
Magnetic cross-linked polyvinylalcohol
beads |
|
Adsorption study |
[76] |
|
|
Commercial preparation |
Magnetic agarose beads |
Cibacron blue 3GA |
Magnetically stabilized fluidized bed |
[77] |
|
|
|
Magnetic chitosan |
Cibacron blue 3GA |
Study of adsorption properties |
[78] |
|
|
Commercial preparation |
Ferrofluid modified sawdust |
|
Elution with 0.5 M NaCl |
[79] |
|
|
Commercial preparation |
Nano-sized magnetic particles |
|
Elution with NaH2PO4
and NaSCN |
[80] |
|
Lysozyme (recombinant, histidine-tailed) |
T4 |
BioMag, amine terminated |
Iminodiacetic acid charged with Cu2+ |
Elution with low pH buffer |
[81] |
Table 3: Examples of polysaccharide and
disaccharide hydrolases purified by magnetic techniques
|
Purified enzyme |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
a-Amylases |
Porcine pancreas, |
Magnetic alginate |
|
Elution with 1 M |
И |
|
|
Bacillus subtilis, wheat germ |
beads |
|
maltose |
|
|
|
Bacillus amyloliquefaciens, porcine
pancreas |
Magnetic alginate microbeads |
|
Elution with 1 M maltose |
[10] |
|
P-Amylase |
Sweet potato |
Magnetic alginate beads |
|
Elution with 1 M maltose |
[55] |
|
P-Galactosidase |
Escherichia coli homogenate |
Silanized magnetite |
p-Aminophenyl-P-D- thiogalactopyranoside |
Elution with borate buffer, pH 10 |
[58] |
|
P-Galactosidase (fusion protein
comprising the DNA-binding lac repressor) |
Bacterial lysate |
Magnetic beads |
DNA containing Escherichia coli lac
operator |
Elution with lactose analogue |
[64] |
|
Glucoamylase |
Aspergillus niger |
Magnetic alginate beads |
|
Elution with 1 M maltose |
[55] |
|
Pectinase |
Commercial preparation |
Magnetic alginate beads |
|
|
[82] |
|
Pullulanase |
Bacillus acidopullulyticus |
Magnetic alginate beads |
|
Elution with 1 M maltose |
[55] |
Table 4: Examples of other enzymes
purified by magnetic techniques
|
Purified enzyme |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Alcohol dehydrogenase |
Yeast homogenate |
Magnetic cross-linked polyvinylalcohol |
Cibacron blue 3GA |
Elution with high salt buffer |
[52] |
|
|
Saccharomyces cerevisiae extract |
|
PEG with bound Cibacron blue |
Magnetic two-phase system |
[53] |
|
Aldolase (recombinant, histidine tagged) |
Pea |
Magnetic core and nickel-silica
composite matrix |
Ni2+ |
Elution with imidazole containing buffer |
[36] |
|
Angiol-TEM-P- lactamase |
Escherichia coli cells extracts |
Magnetic agarose beads |
Iminodiacetic acid charged with Zn2+ |
Elution with low pH buffer |
[56] |
|
Asparaginase |
Escherichia coli homogenate |
Magnetic polyacrylamide gel particles |
D-Asparagine |
Elution with D- asparagine solution |
[58] |
|
Carbonic anhydrase |
Model mixture |
Magnetic agarose beads |
Sulfanilimide |
Elution with high salt buffer |
[14] |
|
Catalase |
Bovine liver, commercial preparation |
Magnetic poly(EGDMA-MAH) beads |
Fe3+ |
Elution with NaSCN solution |
[61] |
|
Cytochrome c |
Horse, Candida krusei |
Amine terminated iron oxide particles |
Iminodiacetic acid charged with Cu2+ |
Binding studies |
[63] |
|
|
Commercial preparation |
Au@magnetic particles |
|
MALDI MS analysis |
[31] |
|
|
Horse heart |
Magnetic agarose beads |
Iminodiacetic acid charged with Cu2+ |
Elution with EDTA containing buffer |
[56] |
|
|
Bovine heart |
Magnetic ion- exchange particles |
|
Protein binding studies |
[12] |
|
Glucose-6-phosphate dehydrogenase |
|
Ferrofluid modified Sepharose 4B |
ADP |
|
[65] |
|
|
Saccharomyces cerevisiae extract |
|
PEG with bound Cibacron blue |
Magnetic two-phase system |
[53] |
|
Hexokinase |
Escherichia coli homogenate |
|
PEG with bound Cibacron blue |
Magnetic two-phase system |
[53] |
|
Lactate dehydrogenase |
Beef heart |
Ferrofluid modified Sepharose 4B |
AMP |
Elution with 1 mM NADH |
[13] |
|
|
Porcine muscle |
Magnetic agarose beads |
Reactive Red 120 |
Column elution with NaCl gradient |
[66] |
|
Lactoperoxidase |
Sweet whey |
Magnetic cation exchanger |
|
HGMS |
[67,68] |
|
Luciferase (histidine- tagged) |
Escherichia coli homogenate |
MagneHis™ system |
Ni2+ |
|
[69,70] |
|
Phosphatase, alkaline |
Human placenta |
Dynabeads M-450 |
Specific antibody |
Activity of bound enzyme measured |
[83] |
|
Phosphatase, alkaline(fusion protein
comprising the DNA- binding lac repressor) |
Bacterial lysate |
Magnetic beads |
DNA containing Escherichia coli lac operator |
Elution with lactose analogue |
[64] |
|
Phosphofructokinase |
Saccharomyces cerevisiae extract |
|
PEG with bound Cibacron blue |
Magnetic two-phase system |
[53] |
|
6-Phosphogluconate dehydrogenase |
|
Ferrofluid modified Sepharose 4B |
ADP |
Elution with 1 mM NADP |
[13] |
|
Thioredoxin(recombinant,
histidine-tagged) |
Escherichia coli |
Magnetic agarose |
Ni-NTA |
Elution with imidazole containing buffer |
[20] |
|
tRNA methionyl synthetase(recombinant,
histidine-tagged) |
Escherichia coli |
MagneHis™ system |
|
Rapid detection and quantitation of
isolated protein |
[85] |
|
Uricase (recombinant, histidine-tailed) |
Bacillus |
Ion-chelating magnetic agarose beads |
Ni2+ |
Elution by cleavage with proteinase K |
[92] |
Magnetic separation procedures can be employed in several
ways. Preparative isolation of the target protein or peptide is usually
necessary if further detailed study is intended. In other cases, however, the
magnetic separation can be directly followed (after elution with an appropriate
buffer) with SDS electrophoresis. Magnetically separated proteins and peptides
can also be used for further mass spectroscopy characterization [30,31]. The
basic principles of magnetic separations can be used in the course of protein
or peptide determination using various types of solid phase immunoassays.
Usually immu-nomagnetic particles directly capture the target analyte, or
magnetic particles with immobilised streptavidin are used to capture the
complex of biotinylated primary antibody and the analyte. The separated analyte
is then determined (usually without elution) using an appropriate method. A
combination of magnetic separation with affinity capillary electrophoresis is
also possible [32].
Enzyme isolation is usually performed using immobilised
inhibitors, cofactors, dyes or other suitable ligands, or magnetic beads
prepared from affinity biopolymers can be used (see Tables 1, 2, 3, 4).
Table 5: Examples of antibodies purified by magnetinetic
techniques
|
Purified antibody |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Anti-BODIPY- fluorescein antibodies |
|
Magnetoliposomes |
BODIPY-fluorescein |
|
[94] |
|
Anti-DNA antibody |
Systemic lupus erythematosus patient
plasma |
Magnetic
poly(2-hydroxyethyl-methacrylate) beads |
DNA |
Desorption with 1 M NaSCN solution |
[95] |
|
Anti-human chorionic gonadotropin
antibody |
Murine ascites supernatants |
Magnetic cellulose beads |
Human chorionic gonadotropin |
|
[96] |
|
Antibody (from rat) |
Sample from affinity chromatography |
Dynabeads M-280 |
Sheep anti-rabbit IgG |
Antibody concentration |
[3] |
|
Antibody |
Rabbit serum |
Dynabeads M-280 |
Sheep anti-rabbit IgG |
Elution with 0.5 M acetic acid |
[97] |
|
Monoclonal antibodies |
Mouse hybridoma culture broth |
Magnetite particles |
Protein A |
|
[98] |
|
Anti-bovine serum albumin antibodies |
|
Thermosensitive magnetic microspheres |
Bovine serum albumin |
Immobilization by the carbodiimide
method |
[99] |
|
Immunoglobulin G, human |
Commercial preparation |
Magnetic poly(ethylene glycol
dimethacrylate-N-methacryloly-L-histidine-methylester) beads |
|
Elution with 1 M NaCl |
[100] |
|
Immunoglobulin G |
Blood serum |
Carboxyl-terminated magnetic particles |
MproteinAG |
|
[101] |
|
IgE antibodies |
Allergic patients sera |
Magnetoliposomes |
Antigenic proteins |
|
[16] |
|
Murine anti-fibroblast growth factor
receptor 1
IgM |
Ascites |
Polystyrene magnetic beads |
Rat anti-mouse IgM monoclonal antibody |
|
[102] |
Genetic engineering enables the construction of gene
fusions resulting in fusion proteins having the combined properties of the
original gene products. To date, a large number of different gene fusion
systems, involving fusion partners that range in size from one amino acid to
whole proteins, capable of selective interaction with a ligand immobilized onto
magnetic particles or chromatography matrices, have been described. In such
systems, different types of interactions, such as enzyme-substrate,
receptor-target protein, polyhistidines-metal ion, and antibody-antigen, have
been utilized. The conditions for purification differ from system to system
and the environment tolerated by the target protein is an important factor for
deciding which affinity fusion partner to choose. In addition, other factors,
including protein localization, costs for the affinity matrix and buffers, and
the possibilities of removing the fusion partner by site-specific cleavage,
should also be considered [33,34]. As an example, isolation of recombinant
oligohistidine-tagged proteins is based on the application of metal chelate
magnetic adsorbents [35,36]. This method has been used successfully for the
purification of proteins expressed in bacterial, mammalian, and insect systems.
Antibodies from ascites, serum and tissue culture
superna-tants can be efficiently isolated using magnetic particles with
immobilized Protein A, Protein G or anti-immunoglobulin antibodies. Protein A, isolated
from Staphylo-coccus aureus, binds the Fc region of IgG of most mammalian
species with high affinity, leaving antigen specific sites free. Protein G,
isolated from Streptococcus sp., reacts with a larger number of IgG isotypes.
It has a higher binding affinity to immunoglobulins than Protein A, however, it
also interacts with the Fab regions of IgG, although the affinity is ten times
lower than for the Fc region [37].
Table 6:
Examples of DNA/RNA/oligonucleotide/aptamer binding proteins purified by
magnetic techniques
|
Purified protein |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
CUG binding proteins |
fibroblasts |
Dynabeads M-280 streptavidin |
Biotinylated(CUG)10 |
Elution with 1 M NaCl |
[103] |
|
Transcription factor x |
Saccharomyces cerevisiae |
Dynabeads M-280 streptavidin |
Biotinylated tRNAGlu gene
fragment |
Elution with high salt buffer |
[104,105] |
|
DNA-binding proteins |
Crude tissue extract |
Magnetic phospho cellulose particles |
|
|
[106] |
|
DNA-binding proteins |
Escherichia coli |
Magnetic phospho cellulose particles |
|
|
[107] |
|
DNA-binding proteins |
HeLa nuclear extracts |
Dynabeads M-280 streptavidin |
Biotin-labelled DNA fragment |
Elution with 2 M NaCl |
[108] |
|
Vaccinia virus early transcription
factor |
Vaccinia virions |
Dynabeads M-280 streptavidin |
Biotinylated double- stranded DNA |
Elution with high salt buffer |
[109] |
|
Ecdysteroid receptor |
Drosophila melanogaster nuclear extract |
Dynabeads M-280 streptavidin |
Biotinylated double- stranded
oligonucleotide |
Elution with 0.4 M KCl |
[110] |
|
NanR protein(recombinant) |
Escherichia coli |
U.MACS streptavidin MicroBeads |
Biotin-labelled DNA fragment |
Elution with 1 M NaCl |
[111] |
|
p27 |
Rabbit hepatocytes |
Dynabeads M-280 streptavidin |
Guanine-rich single- stranded DNA |
Elution with NaCl solution |
[112] |
|
Pigpen protein |
Endothelial cells |
Magnetic streptavidin beads |
Biotinylated aptamer |
Elution with 1 M NaCl |
[113] |
|
RNA binding proteins |
Saccharomyces cerevisiae |
U.MACS streptavidin MicroBeads |
Biotin-labelled RNA probe |
Elution with 1 M NaCl |
[114] |
|
Single-stranded telomere binding protein
(sTBP) |
Nuclei from vertebrate tissues |
Dynabeads M-280 streptavidin |
Biotinylated single stranded TTAGGGn
repeats |
Elution with high salt buffer |
[115] |
|
Transcription proteins |
Human myeloid cells |
Dynabeads M-280 streptavidin |
Biotinylated serum inducible element (hSIE) |
Elution with high salt buffer |
[116] |
|
Transcription factor yRF-l |
Human monocytes and epidermal cells |
Dynabeads M-280 streptavidin |
Biotinylated DNA containing yRF-l
sequences |
Elution with 0.6 M KCl |
[117] |
|
Protein factor MS2 |
Murine skeletal myotubes |
Dynabeads |
Double-stranded DNA |
Elution with 100 mM sodium acetate, pH
4.2 |
[118] |
|
Guide RNA binding protein |
Trypanosoma brucei mitochondria |
Dynabeads M-450 goat anti-mouse IgG |
Monoclonal antibody |
Elution with low pH buffer cont. SDS |
[119] |
|
RNA binding proteins |
Pollen grains |
Streptavidin MagneSphere particles |
Biotinylated oligonucleotides |
Elution with SDS buffer |
[120] |
|
DNA binding protein |
Schistosoma mansoni |
Dynabeads M-280 streptavidin |
Biotinylated DNA |
Elution with sodium acetate buffer |
[121] |
|
ssDNA binding proteins |
Transfected mouse fibroblasts |
Dynabeads anti-rabbit IgG |
Rabbit antibody |
Indirect method |
[122] |
|
Tenascin-C |
Glioblastoma cells |
Dynabeads streptavidin |
Biotinylated aptamer |
Elution with high salt buffer |
[123] |
|
Thermostable brain factor (ThBF) |
Rat brain |
Streptavidin magnetic particles |
Biotinylated oligonucleotides |
Elution with 0.7 M KCl |
[124] |
|
TTF1 protein |
Escherichia coli lysate |
Dynabeads M-280 streptavidin |
Biotinylated aptamer |
Elution with DNase |
[125] |
Antiphospholipid antibodies were successfully isolated
using magnetoliposomes [15].
Aptamers are DNA or RNA molecules that have been
selected from random pools based on their ability to bind other molecules.
Aptamers binding proteins can be immobilised to magnetic particles and used for
isolation of target proteins.
Table 7: Purification of
albumin and haemoglobin by magnetic techniques
|
Purified protein |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Albumin, bovine serum |
Commercial preparation |
Magnetic agar beads |
Cibacron blue3GA |
Adsorption experiments |
[126] |
|
|
Commercial preparation |
Magnetic cross-linked polyvinylalcohol |
Cibacron blue3GA |
Adsorption experiments |
[76,127] |
|
|
|
Magnetic chitosan microspheres |
Cibacron blue3GA |
|
[78] |
|
|
Commercial preparation |
Magnetic poly(glycidyl
methacrylate-triallyl isocyanurate-divinylbenzene) particles |
|
Anion exchange separation |
[128] |
|
|
Commercial preparation |
Magnetic poly(ethylene glycol
dimethacrylate-co-N- methacryloyl-(L)- histidine methylester) microbeads |
Cu2+ |
Elution with 1.0 M NaSCN |
[129] |
|
Albumin, human serum |
Commercial preparation |
Magnetic poly(2-
hydroxyethylmethacrylate) beads |
Iminodiacetic acid charged with Cu2+ |
Elution with 1.0 M NaSCN |
[130] |
|
|
Human plasma |
Magnetic poly(2-hydroxyethyl
methacrylate) beads |
Cibacron blue F3GA |
Elution with 0.5 M NaSCN |
[131] |
|
|
Commercial preparation |
Magnetic particles covered with
thermosensitive polymer |
- |
Desorption by decreasing temperature |
[132,133] |
|
Albumin, human serum (recombinant, FLAG
tagged) |
Yeast cells |
Magnetic glass beads |
Anti-FLAG antibody |
Elution with EDTA containing buffer |
[1] |
|
Glycated haemoglobin |
Human blood |
Magnetic poly(vinyl alcohol) beads |
m-Aminophenyl- boronic acid |
Elution with sorbitol |
[138] |
|
Haemoglobin |
Bovine, commercial preparation |
Amine terminated iron oxide particles |
Iminodiacetic acid charged with Cu2+ |
Elution with imidazole containing buffer |
[63] |
|
Haemoglobin A1cHumanblo |
Human blood |
Magnetic particles isolated from
Magnetospirillum magneticum AMB-1 |
m-Aminophenyl- boronic acid |
used for affinity immunoassay |
[150] |
DNA/RNA binding proteins (e.g., promoters, gene regulatory
proteins and transcription factors) are often shortlived and in low abundance.
A rapid and sensitive method, based on the immobilization of biotinylated
DNA/RNA fragments containing the specific binding sequence to the magnetic streptavidin
particles, can be used. The bound DNA/RNA binding proteins are usually eluted
with high salt buffer or change of pH [38].
Other types of proteins were isolated using specific
affinity-based procedures. For example, plasminogen immobilized on magnetic
particles was used to separate scrapie and bovine spongiform encephalopathy
associated prion protein PrPSc from its conformer which is a
cellular protein called PrPC. In fact, plasminogen represents the
first endogenous factor discriminating between normal and pathological prion
protein. This unexpected property may be exploited for diagnostic purposes
[39,40].
Magnetic separation was also successfully used for the
recovery of proteins expressed in the form of inclusion bodies, involving at
first chemical extraction from the host cells, then adsorptive capture of the
target protein
onto small magnetic adsorbents, followed by rapid
collection of the product-loaded supports with the aid of high gradient
magnetic fields [41].
A new approach for analytical ion-exchange separation
of native proteins and proteins enzymatic digest products has been described
recently [31]. Magnetite particles were covered with a gold layer and then
stabilized with ionic agents. These charged stabilizers present at the surface
of the gold particles are capable of attracting oppositely charged species from
a sample solution through electrostatic interactions. Au@magnetic particles
having negatively charged surfaces are suitable probes for selectively
trapping positively charged proteins and peptides from aqueous solutions. The
species trapped by the isolated particles were then characterized by
matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) after
a simple washing.
Magnetic solid phase extraction (MSPE) enables to
pre-concentrate target analytes from larger volumes of solutions or suspensions
using relatively small amount of magnetic specific adsorbent. Up to now this
procedure was used for preconcentration of low-molecular weight xenobiotics
[42,43] but using suitable magnetic adsorbents the MSPE could be used to
preconcetrate target proteins and peptides as well.
Table 8: Examples of other proteins purified by magnetic
techniques
|
Purified protein |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Aprotinin |
Bovine pancreatic powder |
Magnetic chitosan particles |
Trypsin |
Elution with low pH buffer |
[134] |
|
Concanavalin A |
Jack bean extract |
Magnetic particles |
Dextran |
|
[68,135] |
|
Solanum tuberosum lectin |
Potato tuber |
Magnetic chitosan |
|
Elution with low pH buffer |
[136] |
|
Green fluorescent protein (histidine
tagged) |
|
Magnetic nanoparticles |
Ni-NTA |
Elution with imidazole containing buffer |
[137] |
|
SIRT2 protein(recombinant, histidine
tailed) |
Human |
Magnetic agarose beads |
Ni-NTA |
Elution with imidazole containing buffer |
[139] |
|
Elongation factor(recombinant, histidine
tailed) |
Caenorhabditis elegans |
Magnetic agarose beads |
Ni-NTA |
Elution with imidazole containing buffer |
[140] |
|
Protein A |
Recombinant Escherichia coli |
Magnetic Eudragit |
Human IgG |
Magnetic two-phase system |
[141] |
|
Tumor necrosis factor(TNF) |
|
Dynabeads M-280 |
Mouse monoclonal antibody |
Solid phase immunoassay |
[142] |
|
Anti-MUC1 diabody fragment |
Recombinant Escherichia coli |
Magnetic agarose beads |
Specific peptide |
|
[143] |
|
MHC class II molecules |
MDCK cells |
Dynabeads M-450 rat anti-mouse IgG 1 |
Specific antibodies |
Elution with SDS-PAGE buffer |
[144] |
|
Lamin B3 |
Xenopus egg extracts |
Dynabeads |
Specific antibodies |
Elution with 6 M urea |
[44] |
|
6x-His-tagged proteins |
Human fibroblasts |
Magnetic agarose beads |
Ni-NTA |
Elution with imidazole containing buffer |
[145] |
|
Estrogen receptor |
Adipose tissue |
Dynabeads M-280 streptavidin |
Biotinylated monoclonal mouse anti-human
estrogen receptor antibody |
Indirect method |
[146] |
|
Thiol-reactive chromatin restriction
fragments |
Mouse fibroblasts |
Mercurated agarose magnetic beads |
p- Hydroxymercuribenzoat e |
Elution with 0.5 M NaCl and 20 mM
dithiothreitol |
[147] |
|
L1 coat protein |
Human papillomavirus |
Magnetic polyglutaraldehyde particles |
Iminodiacetic acid charged with Cu2+ |
Elution with imidazole containing buffer |
[41] |
|
Insulin receptor |
Rat muscle or liver extract |
Dynabeads M-450 |
Anti-P5 antibody |
SDS PAGE analysis |
[148] |
|
Stat3 |
DER cells |
Dynabeads |
Biotinylated tyrosine phosphorylated
peptides |
SDS PAGE analysis |
[149] |
|
Transferrin receptor |
Human |
Dynabeads M-450 sheep anti-mouse IgG 1 |
Anti-human transferrin receptor
monoclonal antibody |
SDS analysis |
[151] |
|
Prion protein PrPSc |
Brain extract |
Dynabeads M-280 tosyl activated |
Plasminogen |
SDS analysis |
[39,40] |
|
Biotinylated proteins from extracellular
matrix |
Bipolaris sorokiniana |
Dynabeads |
Streptavidin |
SDS analysis |
[152] |
|
Cryoprotectin |
Leaves of cold- acclimated
cabbage(Brassica oleracea) |
Dynabeads-protein A |
Specific antibody |
|
[153] |
|
Prostate specific antigen |
Serum from a prostate cancer suffering
patient |
Streptavidin-coated magnetic beads |
Biotinylated monoclonal antibody |
Elution with low pH solution |
[154,155] |
|
Estrogen receptor |
In vitro translation |
Magnetic beads |
Antibody |
Elution with SDS buffer |
[156] |
|
VHDL receptor |
Helicoverpa zea |
Streptavidin-coated magnetic beads |
VHDL-biotin ligand |
|
[157] |
|
Fructosyllysine-specific binding protein |
U937 cells |
Dynabeads M-280 tosylactivated |
Poly- L- lysi ne-gl ucose conjugate |
Two proteins isolated |
[158] |
|
Ubiquitin (histidine tagged) |
|
Nickel-gold nanorods |
|
Elution with acidic buffer |
[18] |
Table
9: Examples of peptides purified by magnetic techniques
|
Purified peptide |
Source |
Magnetic carrier |
Affinity ligand |
Further details |
Reference |
|
Biotinylated peptides |
Model mixtures |
Dynabeads M-280 streptavidin |
Streptavidin |
Used in MALDI-TOF mass analysis |
[159] |
|
(His)6-Ala-Tyr-Gly |
Synthetic peptide |
Dynabeads M-280 tosyl activated |
Aminocaproic nitrilotriacetic acid
charged with Ni2+ |
Elution with imidazole solution |
[160] |
|
Synthetic pentapeptides against
fructose-1,6- biphosphate aldolase |
Synthetic mixture |
Streptavidin-coated magnetic beads |
Biotin labelled fructose-1,6-biphosphate
aldolase of
T. brucei |
Pentapeptides were anchored on
polystyrene beads |
[161] |
|
Tryptic digest products of cytochrome c |
Trypsin digested cytochrome c |
Au@magnetic particles |
- |
Ion-exchange separation followed by
MALDI MS analysis |
[31] |
|
Glutathione |
|
Gold and iron oxide nanocomposites |
|
|
[162] |
|
Nisin Z |
Lactobacillus lactis |
EDC activated magnetic beads |
Anti-nisin antibody |
Elution with 6 M urea |
[163] |
Sometimes the removal of certain proteins will reveal
functions involving the depleted proteins or will help in the course of
subsequent protein isolation. As an example, Dynabeads have been used to remove
involved proteins from Xenopus egg extracts for analyses of the cell mitosis mechanisms
[44,45]. Rapid removal of contaminating proteolytic enzymes from the crude
samples could increase yields of sensitive proteins due to the limitation of
their pro teolysis [46].
A combination of mechanical cell disintegration and
magnetic batch affinity adsorption was used to simplify the isolation of
intracellular proteins. Magnetic glass beads were used because of their
hardness and rigidity [1].
An example of quite different protein purification
strategy can also be mentioned. Proteins associated with the endo-cytic vesicles of Dictyostelium discoideum
were separated after
magnetic isolation of the vesicles that was accomplished by feeding the
amoebae with dextran-stabilized iron oxide particles. The cells were broken,
the labelled vesicles were magnetically separated and then disrupted to release
proteins which were resolved by SDS-PAGE. After „in-gel" digestion with
endoproteinase Lys-C or Asp-N the generated peptides were used for amino acid
sequencing. This strategy allowed the identification of the major protein
constituents of the vesicles [47]. Analogous procedure was used for the
separation and study of perox-isomes proteins when at first peroxisomes were
separated using magnetic beads with immobilized specific antibodies and then
the protein content of the separated peroxisomes was analysed [48].
Conclusions
Standard liquid column chromatography is currently the
most often used technique for the isolation and purification of target
proteins and peptides. Magnetic separation techniques are relatively new and
still under development. Magnetic affinity particles are currently used mainly
in molecular biology (especially for nucleic acids separation), cell biology
and microbiology (separation of target cells) and as parts of the procedures for
the determination of selected analytes using magnetic ELISA and related
techniques (especially determination of clinical markers and environmental
contaminants). Up to now separations in small scale prevail and thus the full
potential of these techniques has not been fully exploited.
It can be expected that further development will be
focused at least on two areas. The first one will be focused on the laboratory
scale application of magnetic affinity separation techniques in biochemistry
and related areas (rapid isolation of a variety of both low- and high-molecular
weight substances of various origin directly from crude samples thus reducing
the number of purification steps) and in biochemical analysis (application of
immu-nomagnetic particles for separation of target proteins from the mixture
followed by their detection using ELISA and related principles). Such a type of
analysis will enable to construct portable assay systems enabling e.g.
near-patient analysis of various protein disease markers. New methodologies,
such as the application of chip and microfluidics technologies, may result in
the development of magnetic separation processes capable of magnetic
separation and detection of extremely small amount of target biologically
active compounds [49].
In the second area, larger-scale (industrial) systems
are believed to be developed and used for the isolation of biologically active
compounds directly from crude culture media, wastes from food industry etc.,
integrating three classical steps (clarification, concentration and initial
purification) into a single unit operation [50]. It is not expected that
extremely large amounts of low cost products will be isolated using magnetic
techniques, but the attention should be focused onto the isolation of minor,
but highly valuable components present in raw materials. Of course, prices of
magnetic carriers have to be lowered and special types of low-cost,
biotechnology applicable magnetic carriers and adsorbents prepared by simple
and cheap procedures have to become available. The existence of inexpensive and
effective magnetic separators enabling large-scale operations is necessary, as
well.
In the near future quite new separation strategies can
appear. A novel magnetic separation method, which utilizes the
magneto-Archimedes levitation, has been described recently and applied to
separation of biological materials. By using the feature that the stable
levitation position under a magnetic field depends on the density and magnetic
susceptibility of materials, it was possible to separate biological materials
such as haemoglobin, fibrin-ogen, cholesterol, and so on. So far, the
difference of magnetic properties was not utilized for the separation of
biological materials. Magneto-Archimedes separation may be another way for
biological materials separation [51].
It can be expected that magnetic separations will be
used regularly both in biochemical laboratories and biotechnology industry in
the near future.
Acknowledgements
The research is a part of ILE Research Intention No.
AV0Z6087904. The work was supported by the Ministry of Education of the Czech
Republic (Project No. ME 583) and Grant Agency of the Czech Academy of Sciences
(Project No. IBS6087204).
References
- Schuster
M, Wasserbauer E, Ortner C, Graumann K, Jungbauer A, Hammerschmid F,
Werner G: Short cut of protein purification by integration of
cell-disrupture and affinity extraction. Bioseparation
2000, 9:59-67.
- Hofmann
I, Schnolzer M, Kaufmann I, Franke WW: Symplekin, a constitutive protein of karyo- and cytoplasmic particles involved in mRNA biogenesis in Xenopus laevis
oocytes. M ol В/о/Cell2002, 13:1665-1676.
- AlcheJD,
Dickinson K: Affinity chromatographic purification of antibodies to a biotinylated fusion protein expressed in
Escherichia coli. Protein Expr Purif 1998, 12:1 38-143.
- Teotia
S, Gupta MN: Purification of alpha-amylases using magnetic alginate
beads. Appl Biochem Biotechnol 2001, 90:21 1-220.
- Safarik
I, Safarikova M: Use of magnetic techniques for the isolation of cells.J
Chromatogr B Biomed Sci Appl 1999, 722:33-53.
- Sinclair
B: To bead or not to bead: Applications of magnetic bead technology.
Scientist 1998, 12:17.
- Bruce
IJ, Taylor J, Todd M, Davies MJ, Borioni E, Sangregorio C, Sen T:
Synthesis, characterisation and application of silica-magnetite
nanocomposites. J Magn Magn Mater 2004, 284:145-160.
- Weetall HH,
Lee MJ: Antibodies immobilized on
inorganic supports. Appl Biochem Biotechnol 1989, 22:3 1 1 -330.
- Safarik
I, Safarikova M: Batch isolation of hen egg white lys-ozyme with magnetic
chitin. J Biochem Biophys Methods
1993, 27:327-330.
- Safarikova
M, Roy I, Gupta MN, Safarik I: Magnetic alginate micro-particles for
purification of a-amylases.
J Biotechnol 2003, 105:255-260.
- I
1. Tanyolac D, Ozdural AR: A new low cost magnetic material: magnetic
polyvinylbutyral microbeads. React Funct Polym 2000, 43:279-286.
- Nixon
L, Koval CA, Noble RD, Slaff GS: Preparation and characterization of
novel magnetite-coated ion-exchange particles. Chem Mater 1992,4:117-121.
- I
3. Mosbach K, Andersson L: Magnetic ferrofluids for preparation of
magnetic polymers and their application in affinity chromatography. Nature
1977, 270:259-261.
- Hirschbein
BL, Whitesides GM: Affinity separation of enzymes from mixtures containing
suspended solids. Comparisons of magnetic and nonmagnetic techniques. Appl
Biochem Biotechnol 1982,7:157-176.
- Rocha
FM, de Pinho SC, Zollner RL, Santana MHA: Preparation and characterization
of affinity magnetoliposomes useful for the detection of antiphospholipid
antibodies. J Magn Magn Mater 2001,225:101-108.
- Zollner
TCA, Zollner RD, de Cuyper M, Santana MHA: Adsorption of isotype
"E" antibodies on affinity magnetoliposomes. J Dispersion Sci
Technol 2003, 24:615-622.
- Bucak
S, Jones DA, Laibinis PE, Hatton TA: Protein separations using colloidal
magnetic nanoparticles. Biotechnol Progr 2003, I 9:477-484.
- Lee
KB, Park S, Mirkin CA: Multicomponent magnetic nanorods for biomolecular separations. Angew Chem -
Int Edit 2004, 43:3048-3050.
- Safarik
I, Ptackova L, Safarikova M: Large-scale separation of magnetic
bioaffinity adsorbents. Biotechnol Lett 2001, 23:1953-1956.
- Schafer
F, Romer U, Emmerlich M, Blumer J, Lubenow H, Steinert K: Automated high-throughput purification of 6xHis-tagged proteins. J Biomol Tech 2002, 1 3:1 3 1
-142.
- Lochmuller
CH, Ronsick CS, Wigman LS: Fluidized-bed separators reviewed: a low
pressure drop approach to column chromatography. Prep Chromatogr 1988,
1:93-108.
- Burns
MA, Graves DJ: Continuous affinity
chromatography using a magnetically stabilized fluidized bed. Biotechnol
Progr 1985, 1:95-103.
- 23. Chetty AS, Burns MA: Continuous
protein separations in a magnetically
stabilized fluidized bed
using nonmagnetic
supports. Biotechnol Bioeng 1991, 38:963-971.
- Wikstrom
P, Flygare S, Grondalen A, Larsson PO: Magnetic aqueous two-phase
separation: a new technique to increase rate of phase-separation, using
dextran-ferrofluid or larger iron oxide particles. Anal Biochem 1987,
167:331-339.
- Larsson
P-O: Magnetically enhanced phase separation. Meth Enzymol 1994,228:1 12-1
17.
- Safarik
I, Safarikova M: Biologically active compounds and xeno-biotics: Magnetic
affinity separations. In Encyclopedia of Separation Science Edited by:
Wilson ID, Adlard RR, Poole CF, Cook MR. London: Academic Press; 2000:2163-2170.
- Safarik
I, Safarikova M: Overview of magnetic separations used in biochemical and
biotechnological applications. In
Scientific and Clinical Applications of Magnetic Carriers Edited
by: Hafeli U, Schutt W, Teller J, Zborowski M. New York and London: Plenum
Press; 1997:323-340.
- Safarikova
M, Safarik I: The application of magnetic techniques in biosciences. Magn
Electr Sep 2001, 10:223-252.
- Saiyed
ZM, Telang SD, Ramchand CN: Application of magnetic techniques in the
field of drug discovery and biomedicine. BioMagn Res Technol 2003, 1:2.
- Yaneva
M, Tempst P: Affinity capture of specific DNA-binding proteins for mass
spectrometric identification. Anal
Chem 2003, 75:6437-6448.
- Teng
CH, Ho KC, Lin YS, Chen YC: Gold nanoparticles as selective and concentrating
probes for samples in MALDI MS analysis. Anal Chem 2004, 76:4337-4342.
- 32. Heegaard NHH, Nilsson S, Guzman NA:
Affinity capillary electro-phoresis:
important application areas
and some recent developments. J Chromatogr B
1998, 715:29-54.
- Nilsson
J, Stahl S, LundebergJ, Uhlen M, Nygren PA: Affinity fusion strategies for
detection, purification, and immobilization of recombinant proteins.
Protein Expr Purif 1997, 1 1: 1 -16.
- Kobs
G: Finding the right protein purification system. Cell Notes 2004, 9:2-5.
- 35. Gaberc-Porekar V, Menart V: Perspectives of
immobilized-metal affinity chromatography. J Biochem Biophys Methods 2001,
49:335-360.
- 36. Frenzel A, Bergemann C, Kohl G,
Reinard T: Novel purification system
for 6xHis-tagged proteins by magnetic affinity separation.J Chromatogr B
2003, 793:325-329.
- 37. Widjojoatmodjo MN, Fluit AC, Torensma
R, Verhoef J: Comparison of immunomagnetic beads coated with protein A,
protein G, or
goat anti-mouse immunoglobulins. J Immunol Methods 1993, 165:1 1-19.
- Biomagnetic Techniques in Molecular
Biology. Information Booklet,
Dynal, Oslo, Norway 1998.
- Fischer
MB, Roeckl C, Parizek P, Schwarz HP, Aguzzi A: Binding of
disease-associated prion protein to plasminogen. Nature 2000, 408:479-483.
- Maissen
M, Roeckl F, Glatzel M, Goldmann W, Aguzzi A: Plasminogen binds to
disease-associated prion protein of multiple species. Lancet 2001,
357:2026-2028.
- Heeboll-Nielsen
A, Choe WS, Middelberg APJ, Thomas ORT: Efficient inclusion body processing
using chemical extraction and
high gradient magnetic fishing. Biotechnol
Progr 2003, 19:887-898.
- Safarikova
M, Safarik I: Magnetic solid-phase extraction. J Magn Magn Mater 1999,
194:108-1 12.
- Safarikova
M, Safarik I: Magnetic solid-phase extraction of target analytes from
large volumes of urine. Eur Cells Mater 2002, 3(Suppl 2): 192-195.
- Goldberg
M, Jenkins H, Allen T, Whitfield WG, Hutchison CJ:Xeno-pus lamin B3 has a
direct role in the assembly of a replication competent nucleus: evidence
from cell-free egg extracts. J Cell Sci 1995, 108:3451-3461.
- Bell
P, Scheer U: Prenucleolar bodies contain coilin and are assembled in
Xenopus egg extract depleted of specific nucle-olar proteins and U3 RNA.J
Cell Sci 1997, 1 10:43-54.
- Safarik
I, Safarikova M: Isolation and removal of proteolytic enzymes with
magnetic cross-linked erythrocytes. J Magn Magn Mater 2001, 225:169-174.
- Adessi
C, Chapel A, Vincon M, Rabilloud T, Klein G, Satre M, Garin J:
Identification of major proteins associated with Dictyostel-ium discoideum
endocytic vesicles.J Cell Sci 1995, 108:333 1 -3337.
- 48. Luers GH, Hartig R, Mohr H, Hausmann
M, Fahimi HD, Cremer C, Volkl A:
Immuno-isolation of highly purified peroxisomes using magnetic beads and continuous immunomagnetic sorting. Electrophoresis 1998, 1 9:1205-1210.
- Brzeska
M, Panhorst M, Kamp PB, Schotter J, Reiss G, Puhler A, Becker A, Bruckl H:
Detection and manipulation of biomole-cules by magnetic carriers. J
Biotechnol 2004, 1 12:25-33.
- Hubbuch
JJ, Thomas ORT: High-gradient magnetic affinity separation of trypsin
from porcine pancreatin. Biotechnol Bioeng 2002,79:301-313.
- Hirota
N, Kurashige M, Iwasaka M, Ikehata M, Uetake H, Takayama T, Nakamura H,
Ikezoe Y, Ueno S, Kitazawa K: Magneto-Archimedes separation and its application
to the separation of biological materials. Physica B 2004,
346-347:267-271.
- 52. Tong XD, Xue B, Sun Y: A novel
magnetic affinity support for protein
adsorption and purification. Biotechnol Progr 2001, 17:1
34-1 39.
- Flygare
S, Wikstrom P, Johansson G, Larsson PO: Magnetic aqueous two-phase
separation in preparative applications. Enzyme Microb Technol 1990,
12:95-103.
- Murphy
AS, Hoogner KR, Peer WA, Taiz L: Identification, purification, and
molecular cloning of N-1-naphthylphthalmic acid-binding
plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol
2002, 128:935-950.
- 55. Teotia S, Gupta MN:
Magnetite-alginate beads for purification of some starch degrading
enzymes. Mol Biotechnol 2002, 20:231-237.
- Abudiab
T, Beitle RR: Preparation of magnetic immobilized metal affinity
separation media and its use in the isolation of proteins.J Chromatogr A
1998, 795:21 1-217.
- Barnes
K, Murphy LJ, Turner AJ: Immunoseparation of membrane peptidases from pig
lung membranes using magnetic beads. Biochem Soc Trans 1994, 22:S451-S451.
- Dunnill
P, Lilly MD: Purification of enzymes using magnetic bio-affinity
materials. Biotechnol Bioeng 1974, 16:987-990.
- Chen
D-H, Huang S-H: Fast separation of bromelain by poly-acrylic acid-bound
iron oxide magnetic nanoparticles. Process Biochem 2004, 39:2207-221 1.
- Himeji
D, Horiuchi T, Tsukamoto H, Hayashi K, Watanabe T, Harada M:
Characterization of caspase-8L: a novel isoform of cas-pase-8 that behaves
as an inhibitor of the caspase cascade. Blood 2002, 99:4070-4078.
- Akgol
S, Denizli A: Novel metal-chelate affinity sorbents for reversible use in
catalase adsorption.JMol CatalB- Enzym 2004, 28:7-14.
- Ghosh M, Tyagi R, Gupta MN:
Preparation of trypsin free chymotrypsin. Biotechnol Tech
1995, 9:149-152.
- O'Brien
SM, Thomas ORT, Dunnill P: Non-porous magnetic che-lator supports for
protein recovery by immobilised metal affinity adsorption.J Biotechnol
1996, 50:13-25.
- Ljungquist
C, Lundeberg J, Rasmussen AM, Hornes E, Uhlen M:
Immobilization and recovery of fusion proteins and B-lym-phocyte cells
using magnetic separation. DNA Cell Biol 1993, 12:191-197.
- Griffin
T, Mosbach K, Mosbach R: Magnetic biospecific affinity adsorbents for
immunoglobulin and enzyme isolation. Appl Biochem Biotechnol 1981,
6:283-292.
- Ennis
MP, Wisdom GB: A magnetizable solid phase for enzyme extraction. Appl
Biochem Biotechnol 1991, 30:155-164.
- Justesen
SFL, Nielsen AH, Thomas ORT: High gradient magnetic fishing for the
isolation of high-value proteins from sweet whey. In: Danish Biotechnology
Conference VII: Vejle, Denmark. 2001.
- Heeboll-Nielsen
A, Justesen S, Thomas ORT: Product recovery for crude bioprocess liquors
by high gradient magnetic fishing. In: 1 0th European Congress on
Biotechnology: Madrid, Spain. 2001.
- Betz
N: Efficient purification of His-tagged proteins from insect and mammalian
cells. Promega Notes 2004, 87:29-32.
- Betz
N: Purifying His-tagged proteins from insect and mammalian cells. Cell
Notes 2004, 9:6-9.
- Safarik
I: Magnetic biospecific affinity adsorbents for lysozyme isolation.
Biotechnol Tech 1991, 5:1 1 1 -1 14.
- Odabasi
M, Denizli A: Cibacron blue F3GA incorporated magnetic
poly(2-hydroxyethyl methacrylate) beads for lysozyme adsorption.J Appl
Polym Sci 2004, 93:719-725.
- Goto
M, Imamura T, Hirose T: Axial dispersion in liquid magnetically
stabilized fluidized beds.J Chromatogr A 1995, 690:1 -8.
- Kopacek
P, Vogt R, Jindrak L, Weise C, Safarik I: Purification and
characterization of the lysozyme from the gut of the soft tick
Ornithodoros moubata. Insect Biochem Mol Biol 1999, 29:989-997.
- Liao
MH, Chen DH: Fast and efficient adsorption/desorption of protein by a
novel magnetic nano-adsorbent. Biotechnol Lett 2002,24:1913-1917.
- Xue
B, Sun Y: Protein adsorption equilibria and kinetics to a poly(vinyl
alcohol)-based magnetic affinity support.J Chromatogr A 2001, 921:109-1
19.
- 77. Tong XD, Sun Y: Application of
magnetic agarose support in liquid
magnetically
stabilized fluidized bed
for protein adsorption.
Biotechnol Progr 2003, 19:1721 -1727.
- Yu
YH, Xue B, Sun Y, He BL: The preparation of chitosan affinity magnetic
nanoparticles and their adsorption properties for proteins. Acta Polym
Sinica 2000:340-344.
- Safarik
I, Safarikova M, Weyda F, Mosiniewicz-Szablewska W, Slaw-ska-Waniewska A:
Ferrofluid-modified plant-based materials as adsorbents for batch
separation of selected biologically active compounds and xenobiotics.J
Magn Magn Mater in press.
- Peng
ZG, Hidajat K, Uddin MS: Adsorption and desorption of lysozyme on
nano-sized magnetic particles and its conforma-tional changes. Colloid
Surf B - Biointerfaces 2004, 35:169-174.
- O'Brien
SM, Sloane RP, Thomas ORT, Dunnill P: Characterisation of non-porous
magnetic chelator supports and their use to
- recover
polyhistidine-tailed T4 lysozyme from a crude E. coli
- extract.J
Biotechnol 1997, 54:53-67.
- 82. Tyagi R, Gupta MN: Purification and
immobilization of Aspergil-lus
niger pectinase on
magnetic latex beads. Biocatal Biotransform 1995, 12:293-298.
- 83. Hendrix PG, Hoylaerts MF, Nouwen EJ,
Van de Voorde A, De Broe ME: Magnetic beads in suspension enable a rapid
and sensitive
immunodetection of human placental alkaline
phosphatase. EurJ Clin Chem Clin Biochem 1992, 30:343-347.
- HubbuchJJ,
Matthiesen DB, HobleyTJ, Thomas ORT: High gradient magnetic separation
versus expanded bed adsorption: a first principle comparison.
Bioseparation 2001, 10:99-1 12.
- 85. Engel L, Kar S, Johnson T: Rapid
detection and quantitation of His-tagged
proteins purified by MagneHis™ Ni-particles. Promega Notes 2003, 84:27-30.
- Khng
HP, Cunliffe D, Davies S, Turner NA, Vulfson EN: The synthesis of
sub-micron magnetic particles and their use for preparative purification
of proteins. Biotechnol Bioeng 1998, 60:419-424.
- 87. Halling PJ, Dunnill P: Recovery of free enzymes from product
liquors by bio-affinity adsorption: Trypsin binding by immobilised soybean inhibitor.
European J Appl
Microbiol 1979,
6:195-205.
- Lochmuller
CH, Wigman LS: Affinity separations in magnetically stabilized fluidized
beds. Synthesis and performance of packing materials. Sep Sci Technol
1987, 22:21 1 1-2125.
- Lochmuller
CH, Wigman LS, Kitchell BS: Aerosol-jet produced, magnetic carrageenan-gel
particles: a new affinity chroma-tography matrix.J Chem Technol Biotechnol
1987, 40:33-40.
- 90. Cocker TM, Fee CJ, Evans RA:
Preparation of magnetically susceptible polyacrylamide/magnetite beads
for use in magnetically
stabilized fluidized bed
chromatography. Biotechnol
Bioeng 1997,53:79-87.
- 91. An XN, Su ZX: Characterization and
application of high magnetic
property chitosan particles. J Appl Polym Sci
2001, 81:1 175-1181.
- Nishiya
Y, Hibi T, Oda JL: A purification method of the diagnostic enzyme
Bacillus uricase using magnetic beads and nonspecific protease. Protein
Expr Purif 2002, 25:426-429.
- Dong
YS, Liang F, Jin HX, Xi Q, Chang JH: Preparation of novel magnetic
affinity adsorbents and application for purification of urokinase. Chem J
Chin Univ - Chin 2002, 23:101 3-1017.
- Dumitrascu
G, Kumbhar A, Zhou WL, Rosenzweig Z: The use of derivatized
magnetoliposomes for extraction of antibodies from aqueous solutions. IEEE
Trans Magn 2001, 37:2932-2934.
- 95. Odabasi M, Denizli A:
Polyhydroxyethylmethacrylate-based magnetic DNA-affinity beads for anti-DNA antibody removal from systemic lupus
erythematosus patient plasma. J Chromatogr B 2001, 760:137-148.
- Li
X, Li CX, He BL: Preparation and application of magnetic affinity
adsorbent based on magnetic cellulose bead. Chem J Chin Univ - Chin 1998,
19:994-999.
- 97. Quitadamo IJ, Kostman TA, Schelling
ME, Franceschi VR: Magnetic bead
purification as a
rapid and efficient method for
enhanced antibody specificity for plant sample immunoblot-ting and
immunolocalization. Plant Sci 2000, 1 53:7-14.
- Shinkai
M, Kamihira M, Honda H, Kobayashi T: Rapid purification of monoclonal
antibody with functional magnetite particles. Kag Kog Ronbunshu 1992, 1
8:256-259.
- Kondo
A, Kamura H, Higashitani K: Development and application of
thermo-sensitive magnetic immunomicrospheres for antibody purification.
Appl Microbiol Biotechnol 1994, 41:99-105.
- Ozkara
S, Akgol S, Canak Y, Denizli A: A novel magnetic adsorbent for
immunoglobulin-G purification in a magnetically stabilized fluidized bed.
Biotechnol Progress 2004, 20:1 169-1 175.
- Meng
ZH, Lin B, Xie YH, Zhang KQ: Cloning and expression of fused Fc-binding
protein (SPA-SPG) and its application in purification of IgG. Progr
Biochem Biophys 2004, 31:146-149.
- Quitadamo
IJ, Schelling ME: Efficient purification of mouse anti-FGF receptor IgM
monoclonal antibody by magnetic beads. Hybridoma 1998, 17:199-207.
- 103. Bhagwati S, Ghatpande A, Leung B:
Identification of two nuclear proteins which bind to RNA CUG repeats: Significance
for myotonic dystrophy. Biochem Biophys Res Commun 1996, 228:55-62.
- Gabrielsen
OS, Hornes E, Korsnes L, Ruet A, Oyen TB: Magnetic DNA affinity
purification of yeast transcription factor x - a
- new
purification principle for the ultrarapid isolation of near homogeneous
factor. Nucleic Acids Res 1989, 1 7:6253-6267.
- Gabrielsen
OS, Huet J: Magnetic DNA affinity purification of yeast transcription
factor. Meth Enzymol 1993, 21 8:508-525.
- 106. Hollung K, Gabrielsen OS, Jakobsen KS:
Enrichment of DNA-binding
proteins from crude tissue for electrophoretic mobility shift assay using magnetic phospho cellulose
particles. Nucleic Acids Res 1994, 22:3261-3262.
- 107. Risoen PA, Struksnes K, Myrset AH,
Gabrielsen OS: One-step magnetic
purification of
recombinant DNA-binding proteins using magnetizable
phosphocellulose. Protein Expr Purif 1995, 6:272-277.
- Zeng
GC, Gao LY, Xia T, Tencomnao T, Yu RK: Characterization of the 5'-flanking fragment of the human
GM3-synthase gene. Biochim Biophys Acta 2003, 1625:30-35.
- Gershon
PD, Moss B: Early transcription factor subunits are encoded by vaccinia
virus late genes. Proc Natl Acad Sci USA 1990,87:4401-4405.
- I
10. Ozyhar A, Gries M, Kiltz HH, Pongs O: Magnetic DNA affinity purification
of ecdysteroid receptor. J Steroid Biochem Mol Biol 1992,43:629-634.
- Kalivoda
KA, Braun KW, Vimr ER: Rapid purification of a prokaryotic regulatory
protein with U.MACS™ Streptavidin MicroBeads. MACS&more 2003, 7:8-9.
- I
12. Rahat MA, Fry M: Purification and characterization of p27, a protein
from hepatocyte chromatin. Evidence suggesting that it binds selectively
to guanine-rich single-stranded DNA. FEBS Lett 1993, 334:60-64.
- I
1 3. Blank M, Weinschenk T, Priemer M, Schluesener H: Systematic evolution
of a DNA aptamer binding to rat brain tumor microvessels. Selective
targeting of endothelial regulatory protein pigpen. J Biol Chem 2001,
276:16464-16468.
- I
14. Albig A: Isolation of mRNA binding proteins using the U.MACS
streptavidin kit. MACS&more 2001, 5:6-7.
- I
15. McKay SJ, Cooke H: A protein which specifically binds to single
stranded TTAGGGn repeats. Nucleic Acids Res 1992, 20:1387-1391.
- I
16. Chakraborty A, White SM, Schaefer TS, Ball ED, Dyer KF, Tweardy DJ:
Granulocyte colony-stimulating factor activation of Stat3a and Stat3(3 in
immature normal and leukemic human myeloid cells. Blood 1996, 88:2442-2449.
- I
17. Feghali CA, Wright TM: Ligand-dependent and ligand-inde-pendent
activation of the transcription factor Rf-I in a cell-free system. Biochem
J 1995, 310:461-467.
- I
18. Ren LF, Chen H, Sternberg EA: Tethered bandshift assay and affinity
purification of a new DNA-binding protein. Biotech-niques 1994,
16:852-855.
- I
19. Allen TE, Heidmann S, Reed R, Myler PJ, Goringer HU, Stuart KD:
Association of guide RNA binding protein gBP21 with active RNA editing
complexes in Trypanosoma brucei. Mol Cell Biol 1998, 18:6014-6022.
- Honys
D: Isolation of proteins comprising native gene-specific messenger
ribonucleoprotein particles using paramagnetic beads. Plant Sci 2001,
161:605-61 1.
- Fantappie
MR, Correa-Oliveira R, Caride EC, Geraldo EA, Agnew A, Rumjanek FD:
Comparison between site-specific DNA binding proteins of male and female
Schistosoma mansoni. Comp Biochem Physiol B - Biochem Mol Biol 1999,
124:33-40.
- 122. Kelm RJ Jr, Cogan JG, Elder PK,
Strauch AR, Getz MJ: Molecular interactions between single-stranded
DNA-binding proteins associated with an essential MCAT element in the
mouse smooth muscle alpha-actin promoter. J
Biol Chem 1999, 274:14238-14245.
- Daniels
DA, Chen H, Hicke BJ, Swiderek KM, Gold L: A tenascin-C aptamer identified
by tumor cell SELEX: Systematic evolution of ligands by exponential
enrichment. Proc Natl Acad Sci USA 2003, 100:15416-15421.
- Skala-Rubinson
H, Vinh J, Labas V, Kahn A, Tuy FPD: Novel target sequences for Pax-6 in
the brain-specific activating regions of the rat aldolase C gene.J Biol
Chem 2002, 277:47190-47196.
- Murphy
MB, Fuller ST, Richardson PM, Doyle SA: An improved method for the in
vitro evolution of aptamers and applications in protein detection and
purification. Nucleic Acids Res 2003, 31 :e 1 10.
- Tong
XD, Sun Y: Agar-based magnetic affinity support for protein adsorption.
Biotechnol Progr 2001, 17:738-743.
- Xue
B, Tong XD, Sun Y: Characterization of PVA-based magnetic affinity
support for protein adsorption. Sep Sci Technol 2001,36:2449-2461.
- Xue
B, Sun Y: Fabrication and characterization of a rigid magnetic matrix for protein adsorption. J Chromatogr A 2002, 947:185-193.
- Akgol
S, Turkmen D, Denizli A: Cu(II)-incorporated, histidine-containing, magnetic-metal-complexing beads
as specific sorbents for
the metal chelate affinity of albumin. J Appl Polym Sci 2004,
93:2669-2677.
- 130. Odabasi M, Uzun L, Denizli A: Porous
magnetic chelator support for
albumin adsorption by
immobilized metal affinity separation. J Appl Polym Sci
2004, 93:2501 -2510.
- Odabasi
M, Denizli A: Cibacron Blue F3GA-attached magnetic poly(2-hydroxyethyl
methacrylate) beads for human serum albumin adsorption. Polym Int 2004,
53:332-338.
- Ding
XB, Sun ZH, Zhang WC, Peng YX, Wan GX, Jiang YY: Adsorp-tion/desorption of
protein on magnetic particles covered by thermosensitive polymers. J Appl
Polym Sci 2000, 77:2915-2920.
- DingXB,
SunZH, Wan GX, Jiang YY: Interactions between thermosensitive magnetic
polymer microspheres and proteins. Acta Polym Sinica 2000:9-1 3.
- 134. An XN, Su ZX, Zeng HM: Preparation of
highly magnetic chi-tosan
particles and their
use for affinity purification of enzymes. J Chem Technol Biotechnol 2003, 78:596-560.
- Heeboll-Nielsen
A, Dalkiaer M, Hubbuch JJ, Thomas ORT: Super-paramagnetic adsorbents for
high-gradient magnetic fishing of lectins
out of legume extracts. Biotechnol
Bioeng 2004, 87:31 1-323.
- Safarikova
M, Safarik I: One-step partial purification of Solanum tuberosum tuber
lectin using magnetic chitosan particles. Biotechnol Lett 2000, 22:941
-945.
- Xu
CJ, Xu KM, Gu HW, Zhong XF, Guo ZH, Zheng RK, Zhang XX, Xu B:
Nitrilotriacetic acid-modified magnetic nanoparticles as a general agent
to bind histidine-tagged proteins. J Am Chem Soc 2004, 126:3392-3393.
- Muller-Schulte
D, Brunner H: Novel magnetic microspheres on the basis of poly(vinyl
alcohol) as affinity medium for quantitative detection of glycated
hemoglobin. J Chromatogr A 1995, 71 1:53-60.
- Dryden
SC, Nahhas FA, NowakJE, Goustin A-S, Tainsky MA: Role for human SIRT2
NAD-dependent deacetylase activity in control of mitotic exit in the cell
cycle. Mol Cell Biol 2003, 23:3173-3185.
- Ohtsuki
T, Sakurai M, Sato A, Watanabe K: Characterization of the interaction
between the nucleotide exchange factor EF-Ts from nematode mitochondria and
elongation factor Tu. Nucleic Acids Res 2002, 30:5444-5451.
- Suzuki
M, Kamihira M, Shiraishi T, Takeuchi H, Kobayashi T: Affinity partitioning
of protein A using a magnetic aqueous two-phase system. J Ferment Bioeng
1995, 80:78-84.
- Liabakk
NB, Nustad K, Espevik T: A rapid and sensitive immu-noassay for tumor
necrosis factor using magnetic monodis-perse polymer particles. J Immunol
Methods 1990, 134:253-259.
- Zhang
ZR, O'Sullivan DA, Lyddiatt A: Magnetically stabilised flu-idised bed
adsorption: practical benefit of uncoupling bed expansion from fluid
velocities in the purification of a recom-binant protein from Escherichia
coli. J Chem Technol Biotechnol 1999,74:270-274.
- Simonsen
A, Stang E, Bremnes B, Roe M, Prydz K, Bakke O: Sorting of MHC class II
molecules and the associated invariant chain (Ii) in polarized MDCK
cells.J Cell Sci 1997, 1 10:597-609.
- Mikaelsdottir
EK, Valgeirsdottir S, Eyfjord JE, Rafnar T: The Icelandic founder
mutation BRCA2 999del5: analysis of expression. Breast Cancer Res 2004,
6:R284-R290.
- Welter
BH, Price TM: Magnetic separation to concentrate the estrogen receptor
from adipose tissue for western analysis. Biotechniques 1999, 27:282-286.
- 147. Chen-Cleland TA, Boffa LC, Carpaneto
EM, Mariani MR, Valentin E, Mendez
E, Allfrey VG: Recovery of transcriptionally active chromatin
restriction fragments by binding to organomer-curial-agarose magnetic
beads. A rapid and sensitive method for monitoring changes in higher order
chromatin structure during
gene activation and
repression. J Biol Chem
1993, 268:23409-23416.
- Knutson
VP, Donnelly PV, Balba Y, Lopez-Reyes M: Insulin resistance is mediated
by a proteolytic fragment of the insulin receptor. J Biol Chem 1995,
270:24972-24981.
- Chakraborty
A, Dyer KF, Cascio M, MietznerTA, Tweardy DJ: Identification of a novel
Stat3 recruitment and activation motif within the granulocyte
colony-stimulating factor receptor. Blood 1999,93:15-24.
- Tanaka
T, Matsunaga T: Detection of HbA(1 c) by boronate affinity immunoassay
using bacterial magnetic particles. Biosens Bioelectron 2001,
16:1089-1094.
- Karlsson
GB, Platt FM: Analysis and isolation of human transfer-rin receptor using
the OKT-9 monoclonal antibody cova-lently cross-linked to
magnetic beads. Anal
Biochem 1991, 199:219-222.
- Apoga
D, Ek B, Tunlid A: Analysis of proteins in the extracellular matrix of
the plant pathogenic fungus Bipolaris sorokini-ana using 2-D gel
electrophoresis and MS/MS. FEMS Microbiol Lett 2001, 197:145-150.
- Hincha
DK, Neukamm B, Sror HAM, Sieg F, Weckwarth W, Ruckels M, Lullien-Pellerin
V, Schroder W, Schmitt JM: Cabbage cryopro-tectin is a member of the
nonspecific plant lipid transfer protein gene family. Plant Physiol 2001,
1 25:835-846.
- Peter
J, Unverzagt C, Lenz H, Hoesel W: Purification of prostate-specific
antigen from human serum by indirect immunosorp-tion and elution with a
hapten. Anal Biochem 1999, 273:98-104.
- Peter
J, Unverzagt C, KroghTN, Vorm O, Hoesel W: Identification of precursor
forms of free prostate-specific antigen in serum of prostate cancer
patients by immunosorption and mass spectrometry. Cancer Res
2001,61:957-962.
- Yang
Y-S, Yang M-CW, Wang B, Weissler JC: BR22, a novel protein, interacts
with thyroid transcription factor-1 and activates the human surfactant
protein B promoter. Am J Respir Cell Mol Biol 2001, 24:30-37.
- Persaud
DR, Yousefi V, Haunerland N: Efficient isolation, purification, and
characterization of the Helicoverpa zea VHDL receptor. Protein Expr Purif
2003, 32:260-264.
- Salazar
R, Brandt R, Kellermann J, Krantz S: Purification and characterization of
a 200 kDa fructosyllysine-specific binding protein from cell membranes of
U937 cells. Glycoconjugate J 2000, 17:713-716.
- Girault
S, Chassaing G, Blais JC, Brunot A, Bolbach G: Coupling of MALDI-TOF mass
analysis to the separation of biotinylated peptides by magnetic
streptavidin beads. Anal Chem 1996, 68:2122-2126.
- Ji
Z, Pinon DI, Miller LJ: Development of magnetic beads for rapid and
efficient metal-chelate affinity purifications. Anal Biochem
1996,240:197-201.
- 161. Samson I, Rozenski J,
Vanaerschot A, Samyn B, Vanbeeumen J, Herdewijn P:
Screening of a synthetic pentapeptide library composed of D-amino acids against fructose-1,6-biphos-phate aldolase. Lett
Pept Sci 1995, 2:259-260.
- Seino
S, Kinoshita T, Otome Y, Nakagawa T, Okitsu K, Nakayama T, Sekino T,
Niihara K, Yamamoto TA: Radiation-induced synthesis of Au/Fe oxide
nanocomposite particles for magnetic separation of biomolecules. J Ceram
Process Res 2004, 5:1 36-1 39.
- Prioult
G, Turcotte C, Labarre L, Lacroix C, Fliss I: Rapid purification of nisin
Z using specific monoclonal antibody-coated magnetic beads. Int Dairy J
2000, 10:627-633.