The preparation of magnetic nanoparticles for applications in
biomedicine
Pedro Tartaj1, Maria del Puerto Morales1,
Sabino Veintemillas-Verdaguer, Teresita Gonzalez-Carreno and Carlos J Serna
Instituto
de Ciencia de Materiales de Madrid, CSIC, Cantoblanco, 28049, Madrid, Spain
E-mail:
ptartaj@icmm.csic.es and puerto@icmm.csic.es
Abstract
This
review is focused on describing state-of-the-art synthetic routes for the
preparation of magnetic nanoparticles useful for biomedical applications. In
addition to this topic, we have also described in some detail some of the
possible applications of magnetic nanoparticles in the field of biomedicine
with special emphasis on showing the benefits of using nanoparticles. Finally,
we have addressed some relevant findings on the importance of having
well-defined synthetic routes to produce materials not only with similar
physical features but also with similar crystallochemical characteristics.
1. Introduction
Nanotechnology
is beginning to allow scientists, engineers, and physicians to work at the
cellular and molecular levels to produce major advances in the life sciences
and healthcare. Real applications of nanostructured materials in life sciences
are uncommon at the present time. However, the excellent properties of these
materials when compared with their bulk counterparts provide a very promising
future for their use in this field [1-3].
Nanoclusters
are ultrafine particles of nanometre dimensions located in the transition
region between molecules and microscopic (micron-size) structures. Viewed as
molecules, they are so large that they provide access to realms of quantum
behaviour that are not otherwise accessible; viewed as materials, they are so
small that they exhibit characteristics that are not observed in larger (even
100 nm) structures. It is in this size regime that many recent advances have
been made in biology, physics, and chemistry [4]. For example, when the
particle dimensions of semiconductor materials become comparable to, or smaller
than the Bohr radius, an increase in the energy band gap is observed
[5-8]. In noble metals, the decrease in size below the electron mean free path
(the distance the electron travels between scattering collisions with the
lattice centres) gives rise to intense absorption in the visible-near-UV region
[9]. Metal nanoparticles also exhibit a broad range of fascinating mechanical
behaviour such as superplasticity [10]. Ceramic materials composed of powders
with a particle size in the nanometric range are also receiving attention
because they may significantly enhance sintering rates or dramatically lower
sintering temperatures [11-14]. Also, ceramic matrix composites with dispersed
nanoparticles have better mechanical properties [10,15].
Magnetic
nanoparticles show remarkable new phenomena such as superparamagnetism, high
field irreversibility, high saturation field, extra anisotropy contributions or
shifted loops after field cooling. These phenomena arise from finite size and
surface effects that dominate the magnetic behaviour of individual
nanoparticles [16]. Frenkel and Dorfman [17] were the first to predict that a
particle of ferromagnetic material, below a critical particle size (< 15
nmfor the common materials), would consist of a single magnetic domain, i.e. a
particle that is a state of uniform magnetization at any field. The
magnetization behaviour of these particles above a certain temperature, i.e.
the blocking temperature, is identical to that of atomic paramagnets
(superparamagnetism) except that an extremely large moment and thus, large
susceptibilities are involved [18].
Industrial
applications of magnetic nanoparticles cover a broad spectrum such as magnetic
seals in motors, magnetic inks for bank cheques, magnetic recording media and
biomedical applications such as magnetic resonance contrast media and
therapeutic agents in cancer treatment [19-22]. Each potential application
requires the magnetic nanoparticles to have different properties. For example,
in data storage applications, the particles need to have a stable, switchable
magnetic state to represent bits of information, a state that is not affected
by temperature fluctuations.
For
biomedical applications the use of particles that present superparamagnetic
behaviour at room temperature (no remanence along with a rapidly changing
magnetic state) is preferred [23-25]. Furthermore, applications in biology and
medical diagnosis and therapy require the magnetic particles to be stable in
water at neutral pH and physiological salinity. The colloidal stability of this
fluid will depend first, on the dimensions of the particles, which should be
sufficiently small so that precipitation due to gravitation forces can be
avoided, and second on the charge and surface chemistry, which give rise to
both, steric and coulombic repulsions [26]. Additional restrictions to the
possible particles that could be used for biomedical applications strongly
depend on whether these particles are going to be used for in vivo or in vitro
applications.
For in
vivo applications the magnetic particles must be coated with a biocompatible
polymer during or after the synthesis process to prevent the formation of large
aggregates, changes from the original structure and biodegradation when exposed
to the biological system. The polymer will also allow binding of drugs by
covalent attachment, adsorption or entrapment on the particles [27, 28]. The
important factors, which determine the biocompatibility and toxicity of these
materials, are the nature of the magnetically responsive component, such as
magnetite, iron, nickel, cobalt, neodimium-iron-boron or samarium-cobalt and
the final size of the particles, their core and the coatings. Iron oxide
particles such as magnetite (Fe3O4) or its oxidized form maghemite (у-Ре2Оз) are by far the most commonly employed for biomedical
applications. Highly magnetic materials such as cobalt and nickel are toxic,
susceptible to oxidation and hence are of little interest [21, 29]. Moreover,
the main advantage of using particles of sizes smaller than 100 nm (so-called
nanoparticles) is their higher effective surface areas (easier attachment of
ligands), lower sedimentation rates (high stability) and improved tissular
diffusion [30]. Another advantage of using nanoparticles is that the magnetic
dipole-dipole interactions are significantly reduced because they scale as r6
(r is the particle radius) [31-33]. Therefore, for in vivo biomedical
applications, magnetic nanoparticles must be made of a non-toxic and
non-immunogenic material, with particle sizes small enough to remain in the
circulation after injection and to pass through the capillary systems of organs
and tissues avoiding vessel embolism. They must also have a high magnetization
so that their movement in the blood can be controlled with a magnetic field and
so that they can be immobilized close to the targeted pathologic tissue [34].
For in
vitro applications the size restrictions are not so severe as in in vivo
applications. Therefore, composites
consisting of superparamagnetic nanocrystals dispersed in submicron diamagnetic
particles with long sedimentation times in the absence of a magnetic field can
be used. The advantage of using diamagnetic matrixes is that the
superparamagnetic composites can be easily provided with functionality.
In almost
all applications the preparation method of the nanomaterials represents one of
the most important challenges that will determine the particle size and shape,
the size distribution, the surface chemistry of the particles and consequently
their magnetic properties. Ferri- and ferromagnetic materials such as Fe3O4,
SrFe12O19, Fe-C and some alloys like SmCo5,
have irregular particle shape when obtained by grinding bulk materials but can
have a spherical shape when prepared by wet chemistry, plasma atomization or
from the aerosol and gas phases. Also, depending on the mechanism of formation,
spherical particles obtained in solution can be amorphous or crystalline if
they result from a disordered or ordered aggregation of crystallites,
respectively. In addition, the preparation method determines to a great extent
the degree of structural defects or impurities in the particle, as well as the
distribution of such defects within the particle and therefore its magnetic
behaviour [16,35].
Recently
many attempts have been made to develop processes and techniques that would
yield 'monodispersed colloids' consisting of uniform nanoparticles both in size
and shape [36-39]. In these systems, the entire uniform physicochemical
properties directly reflect the properties of each constituent particle.
Monodispersed colloids have been exploited in fundamental research and as
models in the quantitative assessment of properties that depend on the particle
size and shape. In addition, it has become evident that the quality and
reproducibility of commercial products can be more readily achieved by starting
with well-defined powders of known properties. In this way, these powders have
found application in photography, inks in high-speed printing, ceramic,
catalysis, and especially in medicine.
The first
part of this review deals with the possible use of magnetic nanoparticles for
biomedical application with special emphasis on the advantage of using
nanoparticles with respect to microparticles. The second part is concerned with
different methods described in the bibliography capable of producing these
magnetic nanoparticles with very narrow particle size distribution, mainly
based on magnetite or maghemite iron oxide nanoparticles [21,29]. Finally, we
address some of the most relevant synthesis effects on the structural and
magnetic properties of the magnetic nanoparticles.
2. Biomedical applications
We can
classify biomedical applications of magnetic nanoparticles according to their
application inside (in vivo) or outside (in vitro) the body. In vivo applications
could be further separated in therapeutic (hyperthermia and drug-targeting) and
diagnostic applications (nuclear magnetic resonance (NMR) imaging), while for
in vitro applications the main use is in diagnostic (separation/selection, and
magnetorelaxometry).
2.1. In vivo
applications
2.1.1.
Therapeutic applications
Hyperthermia.
Hyperthermia is a therapeutic procedure used to raise the temperature of a
region of the body affected by malignancy or other growths. It is administered
together with other cancer treatments (multimodal oncological strategies). The
rationale is based on a direct cell-killing effect at temperatures above
A\-A2°C [40-43]. Modern clinical hyperthermia trials focus mainly on the
optimization of thermal homogeneity at moderate temperatures (42^3°C) in the
target volume. The temperature increase required for hyperthermia can be
achieved, among other methods, by using fine iron oxide magnetic particles
[44]. The physical principle for which a magnetic material can be heated by the
action of an external alternating magnetic field are the loss processes that
occur during the reorientation of the magnetization of magnetic materials with
low electrical conductivity [45,46].
The
advantage of magnetic hyperthermia is that allows the heating to be restricted
to the tumour area. Moreover, the use of subdomain magnetic particles
(nanometre-sized) is preferred instead multidomain (micron-sized) particles
because nanoparticles absorb much more power at tolerable AC magnetic fields
[42, 47-49]. Finally, it should be mentioned that the heating potential is
strongly dependent on the particle size and shape, and thus having well-defined
synthetic routes able to produce uniform particles is essential for a rigorous
control in temperature.
Drug
delivery. Since the pioneering concept proposed by Freeman et al [50] that fine
iron particles could be transported through the vascular system and be
concentrated at a particular point in the body with the aid of a magnetic field
(figure 1), the use of magnetic particles for the delivery of drugs or
antibodies to the organs or tissues altered by diseases has become an
attractive field of research [51,52].
The
process of drug localization using magnetic delivery systems is based on the
competition between forces exerted on the particles by blood compartment, and
magnetic forces generated from the magnet, i.e. applied field. When the
magnetic forces exceed the linear blood flow rates in arteries (10 cms"1)
or capillaries (0.05 cms"1), the magnetic particles are retained
at the target site and maybe internalized by the endothelial cells of the
target tissue [51]. For this application the use of nanoparticles favour the
transport through the capillary systems of organs and tissues avoiding vessel
embolism.
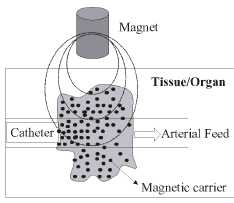
Figure 1. Schematic representation of the magnetically
driven transport of drugs to a specific region. A catheter is inserted into an
arterial feed to the tumour and a magnetic stand is positioned over the
targeted site.
2.1.2.
Diagnostic applications
NMR
imaging. The development of the NMR imaging technique for clinical diagnosis
has prompted the need for a new class of pharmaceuticals, so-called
magneto-pharmaceuticals. These drugs must be administered to a patient in order
to (1) enhance the image contrast between normal and diseased tissue and/or (2)
indicate the status of organ functions or blood flow [53]. A number of
different agents have been suggested as potential NMR contrast agents. Most
contrast agents used in NMR imaging studies to date have been paramagnetic.
Superparamagnetic particles represent an alternative class of NMR contrast
agents that are usually referred to as T2 (transversal relaxation time) or Г2* contrast agents
as opposed to T1 (longitudinal relaxation time) agents such as paramagnetic
Gadolinium(III) chelates [21].
The
relaxation rate increase produced by magnetic particles is a contribution of
several complex mechanisms. The particles possess very large magnetic moments
in the presence of a static magnetic field, and dipolar interactions between
the superparamagnetic cores and surrounding solvent protons result in an
increase in both longitudinal and transverse relaxation rates, especially for
particles with diameters below 10 nm [54-56].
Commercial
iron oxide nanoparticles of maghemite (Endorem® and Resovit®) have been used as
contrast agents in NMR imaging for location and diagnosis of brain and cardiac
infarcts, liver lesions or tumours, where the magnetic nanoparticles tend to
accumulate at higher levels due to the differences in tissue composition
and/or endocytotic uptake processes [57]. Especially promising results have
been detected in the improvement of sensitivity of detection and delineation of
pathological structures, such as primary and metastic brain tumours,
inflammation and ischemia [58]. For this purpose, proteins such as transferrin
[59], peptides such as the membrane traslocating tat peptide of the HIV tat
protein [60,61], and oligonucleotides of various sequences [62] have been attached
to aminated cross-linked iron oxide nanoparticles in order to obtain specific
NMR imaging agents [63].
2.2. In
vitro applications
2.2.1.
Diagnostic applications
Separation and selection. At present,
considerable attention is being paid to solid-phase extraction (SPE) as a way
to isolate and preconcentrate desired components from a sample matrix. SPE
offers an excellent alternative to the conventional sample concentration
methods, such as liquid-liquid extraction [64]. The separation and
preconcentration of the substance from large volumes of solution can be highly
time consuming when using standard column SPE, and is in this field where the
use of magnetic or magnetizable adsorbents called magnetic solid-phase
extraction (MSPE) gains importance. In this procedure the magnetic adsorbent is
added to a solution or suspension containing the target. This is adsorbed onto the magnetic
adsorbent and then the adsorbent with the adsorbed target is recovered from the
suspension using an appropriate magnetic separator (figure 2). For separation
and selection the advantage of using magnetic nanoparticles instead magnetic
microparticles is that we can prepare suspensions that are stable against
sedimentation in absence of an applied magnetic field. The applicability of
iron oxide magnetic nanoparticles in MSPE is clearly evidenced by the fact that
already exists in the market companies (DYNAL Biotech) that commercialize these
products.
Magnetorelaxometry.
Recently, magnetorelaxometry was introduced as a method for the evaluation of
immunoassays [65]. Magnetorelaxometry measures the magnetic viscosity, i.e. the
relaxation of the net magnetic moment of a system of magnetic nanoparticles
after removal of a magnetic field [66]. There are two different relaxation
mechanisms. First, the internal magnetization vector of a nanoparticle relaxes
in the direction of the easy axis inside the core; this is called Neel
relaxation [67]. Second, particles accomplish rotational diffusion in a carrier
liquid, called Brownian relaxation [66]. Neel and Brownian relaxation can be
distinguished by their different relaxation times [68]. Furthermore, Brownian
relaxation can take place only in liquids, whereas Neel relaxation does not
depend on the dispersion of the nanoparticles. The fact that magnetorelaxometry
depends on the core size, the hydrodynamic size and the anisotropy allows this
technique to distinguish between free and bound conjugates by their different
magnetic behaviour, and therefore can be used as an analytical tool for the
evaluation of immunoassays [66]. For this application the benefits of reducing
particle size to the nanometre-sized are similar to those described for
separation and selection applications.
2.3. Future
applications
Magnetically
directed microspheres containing radionucleides have been used for internal
radiotherapy [51]. However, little work has been done in the use of magnetic
nanoparticles in radiotherapy. One
strategy under active investigation to improve dose localization is that of
administration of drugs, metabolites, etc that have been labelled with
radioactive isotopes in a quantity sufficient to deactivate the tumour cells
[69]. In this way, the use of surface-activated magnetic nanoparticles could
have tremendous impact in improving the efficiency of the cancer treatments.
We can
even envisage a future in which magnetic particles could be used for the repair
of the human body with prosthetics or artificial replacement parts. In this
field special mention deserves the pioneering work of Dailey et al [70] who
have reported the synthesis of a silicone based magnetic fluid for use in eye
surgery. Retinal detachment is a major cause of vision loss in adults. It
occurs when the retina separates from the choroid, resulting in eventual death
of the retina and subsequent loss of vision. Dailey and co-workers have
developed an internal tamponade from modified silicone fluid containing
sterically stabilized 4-10 nm sized metal particles, which will be held in
place with an external magnetized scleral buckle.
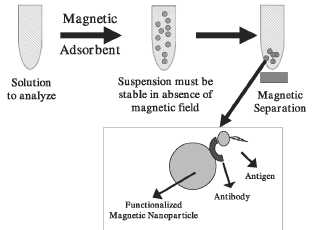
Figure 2. Schematic representation of the magnetically assisted separation of substances. In this particular case a magnetic nanosphere to which an antibody has been anchored is dispersed in a liquid medium containing the antigen (substance to analyse).
3. Synthesis methods
One of the
latest tendencies in materials science is to tailor-make classical products
with controlled properties for special uses. Particular attention should be
paid to the preparation methods that allow the synthesis of particles nearly of
uniform size and shape. This goal can be achieved by precipitation from a
homogeneous solution under controlled conditions or by controlling the particle
growth in a process where a precursor in aerosol or vapour form is decomposed.
Examples of such preparations include gold colloids, sulfur sols, metal oxides
and hydrous oxides [36-38,71,72].
In the
case of magnetic nanoparticles for biomedical applications we have classified
the synthesis methods into those that produce magnetic nanoparticles from
solution techniques or from aerosol/vapour phases, and those producing
composites consisting of magnetic nanoparticles dispersed in submicron-sized
organic or inorganic matrixes that usually have spherical shape. Finally, we
have also described briefly another group of methods that use size selection
principles to produce uniform nanoparticles starting from polydisperse
particles.
3.1.
Magnetic nanoparticles
3.1.1.
Precipitation from solution.
In general
these methods allow the preparation of magnetic nanoparticles with a rigorous
control in size and shape in a simple rather way and thus are very appropriate
for their use in biomedical applications. Uniform particles are usually
prepared via homogeneous precipitation reactions, a process that involves the
separation of the nucleation and growth of the nuclei [38]. A schematic
representation of the different mechanisms proposed in the bibliography to
explain the formation of uniform particles is shown in figure 3.
In a
homogeneous precipitation, a short single burst of nucleation occurs when the
concentration of constituent species reaches critical supersaturation. Then,
the nuclei so obtained are allowed to grow uniformly by diffusion of solutes
from the solution to their surface until the final size is
attained.
To achieve monodispersity, these two stages must be separated and nucleation
should be avoided during the period of growth. This is the classical model
proposed by LaMer and Dinegar [73] first to explain the mechanism of formation
of sulfur colloids and also for a limited number of cases (curve I of figure
3). However, uniform particles have also been obtained after multiple
nucleation events. The uniformity of the final product is in this case achieved
through a self-sharpening growth process (Ostwald ripening, curve III of figure
3) [74]. In addition, uniform particles have also been obtained as a result of
aggregation of much smaller subunits rather than continuous growth by diffusion
(curve II of figure 3) [75-77]. An artificial separation between nucleation and
growth processes may be achieved by seeding in which foreign particles are
introduced into the solution of monomers below the critical supersaturation
[38].
The most
important methods described in the bibliography to obtain uniform iron-based
nanoparticles in solution are briefly described in the following sections:
coprecipitation, microemulsions, the polyol process and decomposition of
organic precursors.
Coprecipitation.
There are two main methods for the synthesis in solution of magnetite spherical
particles in the nanometre range. In the first, ferrous hydroxide suspensions
are partially oxidized with different oxidizing agents [77]. For example,
spherical magnetite particles of narrow size distribution with mean diameters
between 30 and 100 nm can be obtained from a Fe(II) salt, a base and a mild
oxidant (nitrate ions) [77].
The other method consists in ageing
stoichiometric mixtures of ferrous and ferric hydroxides in aqueous media,
yielding spherical magnetite particles homogeneous in size [78]. In addition,
it has been shown that by adjusting the pH and the ionic strength of the
precipitation medium, it is possible to control the mean size of the particles
over one order of magnitude (from 15 to 2 nm) [79]. The size decreases as the
pH and the ionic strength in the medium increases [79]. Both parameters affect
the chemical composition of the surface and consequently, the electrostatic
surface charge of the particles. Under these conditions, magnetite particles
are formed by aggregation of primary particles formed within an Fe(OH)2 gel.
This is an ordered aggregation that gives rise to spherical crystalline
particles [77]. The smallest particles can also be generated after adding
polyvinylalcohol (PVA) to the iron salts [80]. A typical microstructure of
magnetic nanoparticles produced by this method is shown in figure 4.
Modifications
of this method allow for synthesis in the presence of dextran or any other
substance that renders the magnetic nanoparticles biocompatible and thus make
this method especially appropriate for in vivo applications [81,82]. In fact,
this is the most common method used to obtain the commercial NMR contrast
agents based on magnetic nanoparticles. For example, nanosized magnetic
particles are obtained by transferring an acidic iron(II)/iron(III) salt solution
into iron(II,III)-carbonate by adding equivalent amounts of alkaline carbonate,
followed by thermal oxidation. [83] The size of the particles can be controlled
by the thermal reaction velocity and concentration of the iron salts. Thus,
small diameters in the range of 20-100 nm can be obtained by timely separation
of iron(II,III)-carbonate at temperatures of 5-10°C and subsequent heating.
After surplus salts have been removed, the particles can be stabilized with
water-soluble polysaccharide- or synthetic polymer derivatives. Nanoparticles
coated with a starch derivative have a molar mass of 10 kDa. As a result of the
starch matrix, the magnetic particles can retain their dispersion stability in
the pH range 3-12 and also in high salt concentrations [173].
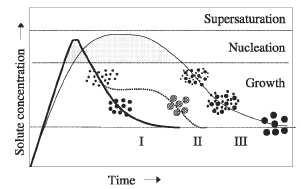
Figure 3. Mechanism of formation of uniform particles in solution: curve I: single nucleation and uniform growth by diffusion (classical model of LaMer and Dinegar); curve II: nucleation, growth and aggregation of smaller subunits; curve III: multiple nucleation events and Ostwald ripening growth.
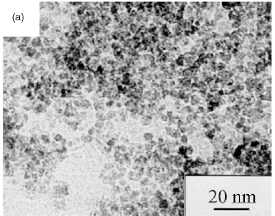
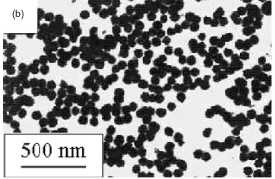
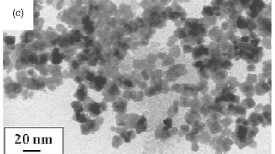
Figure 4. Magnetic nanoparticles prepared in solution
by: (a) coprecipitation (maghemite). (b) Polyol process (Fe-based alloy).
Reprinted from [38]. (c) Microemulsions (maghemite). Reprinted from [91].
Microemulsions.
Water-in-oil (W/O) microemulsions (i.e. reverse micelle solutions) are
transparent, isotropic, thermodynamically stable liquid media. In these
systems, fine microdroplets of the aqueous phase are trapped within assemblies
of surfactant molecules dispersed in a continuous oil phase. The
surfactant-stabilized microcavities (typically in the range of 10 nm) provide a
confinement effect that limits particle nucleation, growth, and agglomeration
[84]. W/O microemulsions have been shown to be an adequate, versatile, and
simple method to prepare nanosized particles [85-90] and these are the
characteristics that could make this method useful for both in vivo and in
vitro applications.
Pileni and
co-workers [91] prepared nanosized magnetic particles with average sizes from 4
to 12nm and standard deviation ranging from 0.2 to 0.3 using microemulsions. A
ferrous dodecyl sulfate, Fe(DS)2, micellar solution was used to produce
nanosized magnetic particles whose size is controlled by the surfactant
concentration and by temperature. A typical microstructure of magnetic
nanoparticles produced by this method is shown in figure 4. Magnetite
nanoparticles around 4 nm in diameter have been prepared by the controlled
hydrolysis with ammonium hydroxide of FeCl2 and FeCl3 aqueous solutions within
the reverse micelle nanocavities generated by using AOT as surfactant and
heptane as the continuous oil phase [92].
Carpenter
and co-workers [93] prepared metallic iron particles coated by a thin layer of
gold via a microemulsion. The gold shell protects the iron core against
oxidation and also provides functionality, making these composites applicable
in biomedicine. The reverse micelle reaction is carried out using
cetyltrimethylammonium bromide (CTAB) as the surfactant, octane as the oil
phase, and aqueous reactants as the water phase [94]. The metal particles are
formed inside the reverse micelle by the reduction of a metal salt using sodium
borohydride. The sequential synthesis offered by reverse micelles is utilized
to first prepare an iron core by the reduction of ferrous sulfate by sodium
borohydride. After the reaction has been allowed to go to completion, the
micelles within the reaction mixture are expanded to accommodate the shell
using a larger micelle containing additional sodium borohydride. The shell is
formed using an aqueous hydrogen tetrachloroaurate solution.
Polyols. A
very promising technique for the preparation of uniform nanoparticles that
could be used in biomedical applications such as magnetic resonance imaging is
the polyol technique. Fine metallic particles can be obtained by reduction of
dissolved metallic salts and direct metal precipitation from a solution
containing a polyol [36,38]. This process was first used to prepare noble
metals such as Ru, Pd, Pt, Au, and others such as Co, Ni or Cu [95,96]. Latterly, the process has been extended to
the synthesis of other materials such as Fe-based alloys [97,98], which could
be used for biomedical applications.
In the
polyol process, the liquid polyol acts as the solvent of the metallic
precursor, the reducing agent and in some cases as a complexing agent for the
metallic cations. The metal precursor can be highly or only slightly soluble in
the polyol. The solution is stirred and heated to a given temperature reaching
the boiling point of the polyol for less reducible metals. By controlling the
kinetic of the precipitation, non-agglomerated metal particles with
well-defined shape and size can be obtained. A better control of the average
size of the metal particles can be obtained by seeding the reactive medium with
foreign particles (heterogeneous nucleation). In this way, nucleation and
growth steps can be completely separated and uniform particles result.
Iron
particles around 100 nm can be obtained by disproportionation of ferrous
hydroxide in organic media [99]. Fe(II) chloride and sodium hydroxide reacts
with ethylene glycol (EG) or polyethylene glycol (PEG) and the precipitation
occurs in a temperature range as low as 80-100°C. Furthermore, iron alloys can
be obtained by coprecipitation of Fe, Ni, and/or Co in EG and PEG.
Monodispersed quasi-spherical and non-agglomerated metallic particles with mean
size around 100 nm have been obtained without seeding (homogeneous nucleation)
while particles between 50 and 100 nm have been obtained using Pt as the
nucleating agent (heterogeneous nucleation). Whereas FeCo particles are formed
by agglomerates of Fe and Co primary particles produced over different lengths
of time, spherical FeNi particles present good homogeneity as a result of
concomitant Fe and Ni formation and growth by the aggregation of nm-sized
primary particles [98]. A typical microstructure of magnetic nanoparticles
produced by the polyol process is shown in figure 4.
High-temperature
decomposition of organic precursors. The decomposition of iron precursors in
the presence of hot organic surfactants has yielded markedly improved samples
with good size control, narrow size distribution and good crystallinity of
individual and dispersible magnetic iron oxide nanoparticles. Biomedical
applications like magnetic resonance imaging, magnetic cell separation or
magnetorelaxometry strongly depend on particle size and thus magnetic
nanoparticles produced by this method could be potentially used for these applications.
For
example, Alivisatos and co-workers [100] have demonstrated that injecting
solutions of FeCup3 (Cup: N-nitrosophenylhydroxylamine) in
octylamine into long-chain amines at 250-300°C yields nanocrystals of
maghemite. These nanocrystals range from 4 to 10 nm in diameter, are
crystalline, and are dispersable in organic solvents (figure 5). Hyeon and
co-workers [101] have also been able to prepare monodisperse maghemite
nanoparticles by a non-hydrolytic synthetic method. For example, to prepare
maghemite nanoparticles of 13 nm (figure 5), Fe(CO)5 was injected into a
solution containing surfactants and a mild oxidant (trimethylamine oxide).
Very
recently, Sun and Zeng [102] have been able to prepare monodispersed magnetite
nanoparticles with sizes from 3 to 20 nm by the high-temperature (265 °C)
reaction of iron(III) acetylacetonate in phenyl ether in the presence of
alcohol, oleic acid, and oleylamine (figure 5). In particular, magnetite
nanoparticles around 4nm were obtained by the thermal decomposition of the iron
precursor but to obtain diameters up to 20 nm a seed-mediated growth method was
required.
Other
solution techniques. Here we describe a series of methods for the production of
magnetic nanoparticles that could be mainly used for in vivo applications. Nature has developed a variety of protein
components that function as carriers or storage devices for metal components.
Of these systems, the iron-storage protein ferritin is probably the most
intensively studied and best understood [ 103]. Ferritin consists of a central
core of hydrated iron(III) oxide encapsulated with a multisubunit protein
shell. As a result of the inner diameter of the nanoreactors, Mann and
co-workers have been able to prepare magnetite [104] and magnetite/maghemite
nanoparticles [105] of about 6-7 nm in diameter. The magnetite/maghemite
particles were generated by oxidation of apoferritin (empty ferritin) with
trimethylamino-N-oxide, which was loaded with various amounts of iron(II) ions.
Of special
interest is the use of dendrimers as templating hosts for the production of
magnetic nanoparticles. In particular, by the judicious selection of the
dendrimers it could be possible to prepare in a single-step biocompatible
magnetic nanoparticles that could be used for in vivo applications. Recently,
iron ferrite nanoparticles have been prepared using dendrimers as templating
hosts [106]. Carboxylated poly(amidoamine) PAMAM dendrimers (generation 4.5)
were utilized for the synthesis and stabilization of ferrimagnetic iron oxide nanoparticles.
Oxidation of Fe(II) at slightly elevated pH and temperature resulted in the
formation of highly soluble nanocomposites of iron oxides and dendrimer, which
are stable under a wide range of temperatures and pHs.
Sonochemical-assisted
synthesis has also been reported as an adequate method for the production of
magnetite and maghemite nanoparticles [107-109]. In sonochemistry, the acoustic
cavitation, that is, the formation, growth, and implosive collapse of a bubble
in an irradiated liquid, generates a transient localized hot spot, with an
effective temperature of 5000 K and a nanosecond lifetime [110]. The cavitation
is a quenching process, and hence the composition of the particles formed is
identical to the composition of the vapour in the bubbles, without phase
separation.
Electrochemical
methods have also been used for the production of maghemite nanoparticles
[111]. The electrochemical synthesis of nanoparticles of y-Fe2O3 was performed
in an organic medium. The size was directly controlled by the imposed current
density, and the resulting particles were stabilized as a colloidal suspension
by the use of cationic surfactants. The size distributions of the particles
were narrow, with the average sizes varying from 3 to 8 nm.
3.1.2.
Aerosol/vapour methods. Spray and laser pyrolysis have been shown to be
excellent techniques for the direct and continuous production of well-defined
magnetic nanoparticles under exhaustive control of the experimental conditions.
Their high-production rate can anticipate a promising future for the
preparation of magnetic nanoparticles useful in in vivo and in vitro
applications. The main difference between spray and laser pyrolysis is the
final state of the ultrafine particles. In spray pyrolysis, the ultrafine
particles are usually aggregated into larger particles, while in laser
pyrolysis the ultrafine particles are less aggregated due to the shorter
reaction time.
Spray
pyrolysis. Spray pyrolysis is a process in which a solid is obtained by
spraying a solution into a series of reactors where the aerosol droplets
undergo evaporation of the solvent and solute condensation within the droplet,
followed by drying and thermolysis of the precipitated particle at higher
temperature [112]. This procedure gives rise to microporous solids, which
finally sinter to form dense particles.
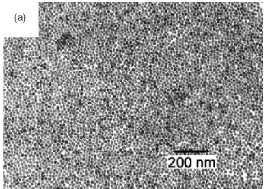
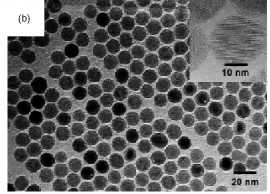
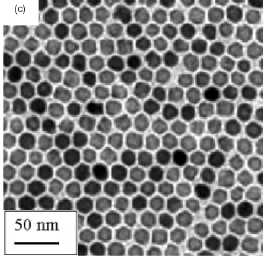
Figure 5. Maghemite nanoparticles prepared in solution
by decomposition at high temperature of organic precursors: (a)FeCup3.
Reprinted from [100]. (b) Fe(CO)5. Reprinted from [101]. (c) Fe(III)
acetylacetonate. Reprinted from [102].
This
method represents a convenient procedure for obtaining finely dispersed
particles of predictable shape, size, and variable composition. The resulting
powders generally consist of spherical particles, the final diameter of which
can be predetermined from that of the original droplets. The method offers
certain advantages over other more commonly used techniques (such as
precipitation from homogenous solution) as it is simple, rapid, and continuous.
Recently, for example has been used for the production of materials with
relevant properties, say mesoporous microspheres [113] and phosphorescent
nanoparticles [114].
Most of
the pyrolysis based processes employed to produce maghemite nanoparticles start
with a Fe3+ salt and some organic compound that acts as the reducing
agent. It was shown that in this procedure Fe3+ is partially reduced
to a mixture of Fe2+ and Fe3+ in the presence of organic
compounds with the formation of magnetite, which is finally oxidized to maghemite.
Without the presence of a reducing agent, hematite is formed instead of
maghemite [115].
In
alcoholic solutions, uniform y-Fe2C>3 particles can be prepared with a wide
variety of particle morphologies and sizes, ranging from 5 to 60 nm, depending
on the nature of the iron precursor salt [116]. A detailed description of the
device used for the preparation of these particles can be found in reference
[117] and a schematic representation is given in figure 6. The device
essentially consists in an aerosol droplet generator (atomizer, ultrasonic,
etc), a furnace and a particle recovery system. Dense aggregates with spherical
shape composed of y-Fe2C>3 subunits with a mean diameter of 6 and 60 nm have
been obtained using Fe(III) nitrate and Fe(III) chloride solutions,
respectively. On the other hand, y-Fe2C>3 obtained from acetylacetonate
solutions resulted in monodispersed particles of about 5 nm in diameter while
maghemite particles derived from Fe(II) ammonium citrate appeared as hollow
spheres with a mean diameter of 300 nm. The latter consisted of small
crystallites aggregated forming a shell, the size of which varied between 10
and 40 nm, depending on the heating temperature in the furnace. A typical
microstructure of magnetic nanoparticles produced by this method is shown in
figure 7.
Laser
pyrolysis. Since the pioneering work of Cannon and co-workers [ 118] on the
continuous production of nanometric powders by laser-induced processes,
different powders such
as Si,
SiC, Si3N4 and a Si/C/N composite have been prepared under a variety of
conditions with sizes ranging from 5 to 20 nm [118, 119]. The method involves
heating a flowing mixture of gases with a continuous wave carbon dioxide laser,
which initiates and sustains a chemical reaction. Above a certain pressure and
laser power, a critical concentration of nuclei is reached in the reaction
zone, which leads to homogeneous nucleation of particles that are further
transported to a filter by an inert gas. Three characteristics of this method
must be emphasized: (a) the small particle size, (b) the narrow particle size
distribution, and (c) the nearly absence of aggregation.
Pure,
well-crystallized and uniform y-Fe2C>3 nanoparticles can be obtained in one
single step by a CO2 laser pyrolysis method (figure 7). Samples with particles
of 3.5 and 5 nm in size and very narrow size distribution have been obtained
under different experimental conditions [120,121]. A schematic representation
of the CO2 laser pyrolysis device used for the preparation of the magnetic
nanocrystals is shown in figure 8. In the device shown in figure 8, a small
reaction zone is defined by the overlap between the vertical reactant gas
stream and the horizontal laser beam. The reaction zone is safely separated
from the chamber walls. This design provides an ideal environment for the
nucleation of small particles in the nanometre range, with less contamination
and narrower size distribution than those prepared by more conventional thermal
methods.
To obtain
the y-Fe2C>3 nanoparticles Fe(CO)5 (iron pentacarbonyl) was used as
precursor. Due to the fact that this precursor does not absorb the radiation at
the laser wavelength (10.60 ± 0.05 /xm), ethylene was used as absorbent as well
as the carrier to transport the carbonyl vapour to the reaction zone. Ethylene
does not decompose at the energy density used (652 W cm"2) but
simply absorbs the laser radiation heating the iron pentacarbonyl, which is
decomposed into iron and carbon monoxide. In order to obtain iron oxide, air
has to be introduced into the system, either with the iron pentacarbonyl vapour
causing oxidation under the laser radiation or mixed with argon.
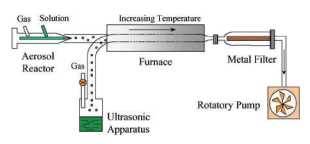
Figure 6. Schematic representation of the spray
pyrolysis device used for the preparation of maghemite nanoparticles. This
device consists of an aerosol generator (atomizer or an ultrasonic bath), one
furnace and a particle recovery system.
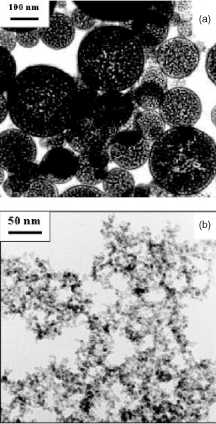
Figure 7. Magnetic nanoparticles of maghemite prepared
by: (a) Spray pyrolysis. (b) Laser pyrolysis. Reprinted from [35].
3.2.
Magnetic composites
For
separation processes i.e. in vitro applications we can use composites
consisting of superparamagnetic nanocrystals dispersed in submicron diamagnetic
matrixes that have long sedimentation times in the absence of a magnetic field.
An advantage of using diamagnetic matrixes is that the superparamagnetic
composite can be easily provided with functionality and biocompatibility. We
now describe some of the most promising methods for the production of
superparamagnetic composites that could be useful in the field of separation.
3.2.1.
Deposition methods.
Inorganic
and hybrid coatings (or shells) on colloidal templates have been prepared by
precipitation and surface reactions [122-126]. By the adequate selection of the
experimental conditions, mainly the nature of the precursors, temperature, and
pH, this method can give uniform, smooth coatings, and therefore lead to
monodispersed spherical composites. Using this technique submicrometre-sized
anionic polystyrene (PS) lattices have been coated with uniform layers of iron
compounds [127, 128] by ageing, at elevated temperature, dispersions of the
polymer colloid in the presence of aqueous solutions of ferric chloride, urea,
hydrochloric acid, and polyvinylpyrrolidone.
One of the
most promising techniques for the production of superparamagnetic composites is
the layer-by-layer (LBL) self-assembly method. This method was firstly
developed for the construction of ultrathin films [129,130] and further
developed by Caruso et al [131, 132] for the controlled synthesis of novel
nanocomposites core-shell materials and hollow capsules. It consists in the
stepwise adsorption of charged polymers or nanocolloids and oppositely charged
polyelectrolytes onto flat surfaces or colloidal templates, exploiting
primarily electrostatic interactions for layer buildup (figure 9).
Using this
strategy, colloidal particles have been coated with alternating layers of
polyelectrolytes, nanoparticles, and proteins [132]. Furthermore, Caruso et al
have demonstrated that submicrometre-sized hollow silica spheres [131] or
polymer capsules [133] can be obtained after removal of the template from the
solid-core multilayered-shell particles either by calcination or by chemical
extraction. Special mention deserves their work in the preparation of iron
oxide superparamagnetic and monodisperse dense and hollow spherical particles
[134,135] that could be used for biomedical applications (figure 10).
3.2.2.
Encapsulation of magnetic nanoparticles in polymeric matrixes.
Encapsulation
of inorganic particles into organic polymers endows particles with important
properties that bare uncoated particles lack [136]. Polymer coatings on
particles enhance compatibility with organic ingredients, reduce susceptibility
to leaching, and protect particle surfaces from oxidation. Consequently,
encapsulation improves dispersibility, chemical stability, and reduces toxicity
[137].
Polymer-coated
magnetite nanoparticles have been synthesized by seed precipitation
polymerization of methacrylic acid and hydroxyethyl methacrylate in the
presence of the magnetite nanoparticles [138]. Cross-linking of polymers has
also been reported an adequate method for the encapsulation of magnetic
nanoparticles. To prepare the composites by this method, first, mechanical
energy needs to be supplied to create a dispersion of magnetite in the presence
of aqueous albumin [139], chitosan [140], or PVA polymers [141]. More energy
creates an emulsion of the magnetic particle sol in cottonseed [139], mineral
[140], or vegetable oil [141]. Depending upon composition and reaction
conditions the addition of a cross-linker and heat results in polydispersed
magnetic colloidal templates. Nanoparticles are adsorbed onto the
polyelectrolyte because they have opposite charge density.
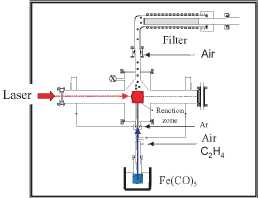
Figure 8. Schematic representation of the Laser pyrolysis device used for the preparation of maghemite nanoparticles around 5 nm
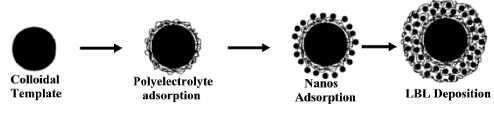
Figure 9. Schematic illustration of the LBL
electrostatic assembly of nanoparticles onto spherical latex, 0.3 microns in
diameter, with up to 24 wt% in magnetite content [139].
Recently,
the preparation of superparamagnetic latex via inverse emulsion polymerization
has been reported [29]. A 'double-hydrophilic' diblock copolymer, present
during the precipitation of magnetic iron oxide, directs nucleation, controls
growth, and sterically stabilizes the resulting 5 nm superparamagnetic iron
oxide. After drying, the coated particles repeptize creating a ferrofluid-like
dispersion. Inverse emulsification of the ferrofluid into decane, aided by small
amounts of diblock copolymer emulsifier along with ultrasonication, creates
minidroplets (180 nm) filled with magnetic particles and monomer. Subsequent
polymerization generates magnetic latex.
A novel
approach to prepare superparamagnetic polymeric nanoparticles by synthesis of
the magnetite core and polymeric shell in a single inverse microemulsion was
reported by Chu and co-workers [142]. Stable magnetic nanoparticle dispersions
with narrow size distribution were thus produced. The microemulsion seed
copolymerization of methacrylic acid, hydroxyethyl methacrylate, and
cross-linker resulted in a stable hydrophilic polymeric shell around the
nanoparticles. Changing the monomer concentration and water/surfactant ratio
controls the particle size.
3.2.3. Encapsulation
of magnetic nanoparticles in inorganic matrixes.
An
appropriate tuning of the magnetic properties is essential for the potential
use of the superparamagnetic composites. In this way, the use of inorganic
matrixes, in particular of silica, as dispersion media of superparamagnetic
nanocrystals has been reported to be an
effective
way to modulate the magnetic properties by a simple heating process [143-145].
Another
advantage of having a surface enriched in silica is the presence of surface
silanol groups that can easily react with alcohols and silane coupling agents
[146] to produce dispersions that are not only stable in non-aqueous solvents
but also provide the ideal anchorage for covalent bounding of specific ligands.
The strong binding makes desorption of these ligands a difficult task. In
addition, the silica surface confers high stability to suspensions of the
particles at high volume fractions, changes in pH or electrolyte concentration
[147].
Recently,
we have been successful in preparing submicronic silica coated maghemite hollow
and dense spheres with a high loading of magnetic material by aerosol pyrolysis
[148,149]. Silica coated у-БегОз hollow spherical particles with an average size of 150 nm (figure 11)
were prepared by the aerosol pyrolysis of methanol solutions containing iron
ammonium citrate and tetraethoxysilane (TEOS) at a total salt concentration of
0.25 M [148]. An illustration of the possible formation mechanism of the silica
coated magnetic hollow spheres is shown in figure 11. During the first stage
the rapid evaporation of the methanol solvent favours the surface precipitation
(i.e. formation of hollow spheres) of components [112]. The low solubility of
the iron ammonium citrate in methanol when compared with that of TEOS promotes
the initial precipitation of the iron salt solid shell. During the second stage
the probable continuous shrinkage of this iron salt solid shell facilitates the
enrichment at the surface of the silicon oxide precursor (TEOS). In the third
stage, the thermal decomposition of precursors produces the silica coated у-ЕегОз hollow spheres.
The formation of the у-ЕегОз is associated with the presence of carbonaceous species coming from the
decomposition of the methanol solvent and from the iron ammonium citrate and
TEOS. On the other hand, the aerosol pyrolysis of iron nitrate and TEOS at a
total salt concentration of 1M produced silica coated y-Fe2O3 dense
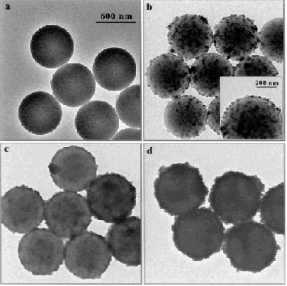
Figure 10. TEM micrographs of uncoated PS particles
(a) and PS particles precoated with a three layer polyelectrolyte film and [Fe3O4/PAH]
(b), [Fe3O4/PAH]4 (c), and [Fe3O4/PDADMAC]4
(d). PAH is a cationic polyelectrolyte (poly(allylamine hydrochloride)) and
PDADMAC is also a cationic polyelectrolyte (poly(diallyldimethylammonium
chloride)). The deposited Fe3O4 nanoparticles can be seen
existing as aggregates. The magnetite loading on the particles increases with
additional depositions of Fe3O4 and poly cation. The
scale bar corresponds to all four TEM images shown. Reprinted from [134].
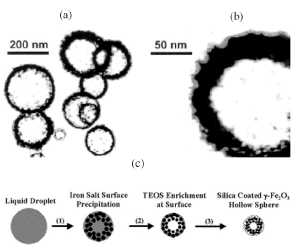
Figure 11. (a) TEM picture of the silica/iron oxide
composites prepared by aerosol pyrolysis of a mixture of iron ammonium citrate
and TEOS. (b) Details of a hollow spherical particle showing an outer particle
layer mainly constituted (according to TEM mycroanalyses) by SiO2. (c)
Illustration of the formation mechanism of the silica coated y-Fe2O3
hollow particles. Reprinted from [148].
3.3. Size
selection methods
Biomedical
applications like magnetic resonance imaging, magnetic cell separation or
magnetorelaxometry utilize the magnetic properties of the nanoparticles in
magnetic fluids. Furthermore, these applications also depend on the
hydrodynamic size. Therefore, in many cases only a small portion of particles
contributes to the desired effect. The relative amount of the particles with
the desired properties can be increased by the fractionation of magnetic fluids
[66,151].
Common
methods currently used for the fractionation of magnetic fluids are
centrifugation [152] and size-exclusion chromatography [153]. All these methods
separate the particles via non-magnetic properties like density or size.
Massart et al [154] have proposed a size sorting procedure based on the
thermodynamic properties of aqueous dispersions of nanoparticles. The positive
charge of the maghemite surface allows its dispersion in aqueous acidic
solutions and the production of dispersions stabilized through electrostatic
repulsions. By increasing the acid concentration (in the range 0.1-0.5
moll"1), interparticle repulsions are screened and phase
transitions are induced. Using this principle, these authors describe a
two-step size sorting process, in order to obtain significant amounts of nanometric
monosized particles with diameters between typically 6 and 13 nm. As the
surface of the latter is not modified by the size sorting process, usual
procedures are used to disperse them in several aqueous or oil-based media.
Preference
should be given, however, to partitions based on the properties of interest, in
this case the magnetic properties. So far, magnetic methods have been used only
for the separation of magnetic fluids, for example, to remove aggregates by
magnetic filtration [155]. Recently, the fractionation of magnetic
nanoparticles by flow field-flow fractionation was reported [156]. Field-flow
fractionation is a family of analytical separation techniques [157], in which
the separation is carried out in a flow with a parabolic profile running
through a thin channel. An external field is applied at a right angle to force
the particles toward the so-called accumulation wall [151].
4. Effect of synthesis on the magnetic properties
4.1.
Particle size and structural effects
We now
present some of our results that clearly manifest the importance of controlling
the particle size and the structure to produce magnetic materials with a
defined magnetic response for a specific biomedical application. It should be
taken into account that size and structural effects are parameters that can be
controlled through the synthesis methods. On the other hand, magnetite and
maghemite are by far the most used materials for biomedical application and
therefore this study is focused on these materials.
Magnetite
has a cubic inverse spinel structure with oxygen forming a fcc close packing
and Fe cations occupying interstitial tetrahedral sites and octahedral sites
[158]. Maghemite has a structure similar to that of magnetite, only differs in
that all or most of the Fe is in the trivalent state (figure 13). Cation
vacancies compensate for the oxidation of Fe(II) cations [158]. Maghemite has a cubic unit cell in
spherical particles with an average size of 250 nm (figure 12). The increase in
salt concentration to a value of 1M favours the formation of dense spherical
particles. Sedimentation studies of these particles have shown that are
particularly useful for separation applications [149].
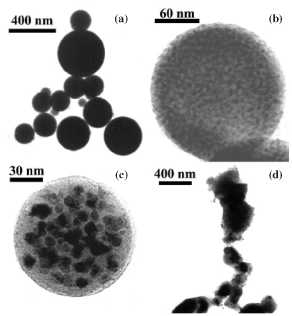
Figure 12. TEM micrographs of the silica/iron oxide composites prepared
by aerosol pyrolysis of a mixture of iron nitrate (20 mol%) and TEOS (a) and
further heated in a conventional furnace for 2 h at 900°C (b), 1050°C (c), and
1200°C (d). Note in the sample heated at 1050°C the presence of y-Fe2C>3
(dark regions) nanoparticles smaller than 20 nm dispersed in a microspherical
silica particle (lighter regions). At this temperature, the enrichment of
silica on particle outerlayers is clearly observed. It is important to note
that similar microstructures to that shown in micrographs (b) and (c) were
observed for smaller and bigger particles. Note also the high stability of the
spherical magnetic composites (the particles lost spherical shape only
temperatures of 1200°C as a consequence of a sintering process). Reprinted from
[149].
A W/O microemulsion method has also been
used for the preparation of silica-coated iron oxide nanoparticles [150]. Three
different non-ionic surfactants (Triton X-100, Igepal CO-520, and Brij-97) have
been used for the preparation of microemulsions, and their effects on the
particle size, crystallinity, and the magnetic properties have been studied.
The iron oxide nanoparticles are formed by the coprecipitation reaction of
ferrous and ferric salts with inorganic bases. A strong base, NaOH, and a
comparatively mild base, NH4OH, have been used with each surfactant to observe
whether the basicity influences the crystallization process during particle
formation. All these systems show magnetic behaviour close to that of
superparamagnetic materials. By use of this method, magnetic nanoparticles as
small as 1-2 nm and of very uniform size (standard deviation less than 10%)
have been synthesized. A uniform silica coating as thin as 1 nm encapsulating
the bare nanoparticles is formed by the base-catalysed hydrolysis and the
polymerization reaction of TEOS in the microemulsion. It is worth mentioning
that the small particle size of the composite renders these particles a
potential candidate for their use in in vivo applications. The cations are
distributed over the 8 tetrahedral and 16 octahedral sites, whereas the
vacancies are confined to the octahedral sites. Synthetic maghemite often
displays superstructure forms, which arises as a result of the cations and the
vacancy ordering. The extent of vacancy ordering is related to both the
crystallite size and the amount of iron(II) in the structure or other
impurities [159]. All of these possible arrangements in the maghemite are
partially responsible for the different magnetic behaviour manifested by
maghemite nanoparticles prepared by different synthetic routes [35].
The extent
of vacancy ordering inside у-БегОз nanoparticles can be easily observed by registering the infrared
spectra of different maghemite samples [35] (figure 14). Thus, in the samples
prepared by solution techniques (coprecipitation), the one with the largest
particle size (14 nm) shows the infrared features of у-БегОз crystallites,
which are at least partially ordered, as evidenced by the multiple lattice
absorption bands between 800 and 200 cm"1. Meanwhile, in the
sample with the lowest particle size (5 nm) a significant reduction in the
number of lattice absorption bands associated with increasing disorder is
detected [ 160]. Noted the difference
in the
infrared spectra of two samples that have a similar particle size (5 nm) but
have been prepared by two different techniques (solution and pyrolysis).
Particularly, the infrared spectrum of the sample prepared by pyrolysis only
displays two broad maximum at around 600 and 450 cm"1
indicating a random distribution of vacancies and therefore is expected to
behave differently in the presence of an applied magnetic field. We also note
from figure 14 that infrared spectroscopy is a simple tool to differentiate
between maghemite and magnetite crystalline phases.
It has
been shown that the degree of order in the distribution of cation vacancies, inherent
in the y-Fe2C>3 structure, of particles smaller than ~100nm affects the
magnetic properties, suggesting that magnetic moments in the interior of the
particles can be significantly influenced by canting effects [161]. For
nanometre y-Fe2Os particles, this effect could explain at least in part the
reduction in saturation magnetization found at very small sizes. In fact, the
existence of magnetically disordered layers around the particles have been
proposed by various researchers as the particle size approaches the frontier of
10 nm [162, 163]. The proposed effects are in many cases, however, obscured by
a wide distribution of particle sizes and shapes or by magnetic interactions
between particles. The effect of the size and structural ordering on the magnetic
properties of y-Fe2C>3 nanoparticles (<20nm) has been carried out in
uniform samples prepared by coprecipitation from solution and laser pyrolysis
methods [35]. The results are shown in figure 15. A progressive cation disorder
that strongly affects the saturation magnetization values is found in
y-Fe2C>3 nanoparticles as the particle size decreases. The smallest
particles, where some vacancy order is observed, are of about 8 nm in diameter.
In general, when the particles are obtained by pyrolysis, the saturation
magnetization is smaller than for samples prepared by precipitation from
solution.
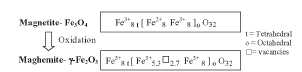
Figure 13. Chemical formula for the magnetite/maghemite system. The order of the vacancies in the octahedral positions of the maghemite can lead to a tetragonal superstructure (unit-cell is three times the cubic one).
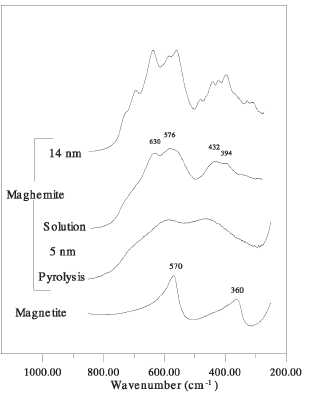
Figure 14. Infrared spectra for magnetite and
maghemite nanoparticles prepared by different methods.
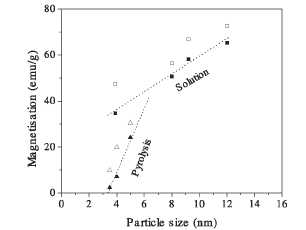
Figure 15. Saturation magnetization values of maghemite nanoparticles as a function of particle size and the preparation method (filled symbols: 298 K, empty symbols: 5 K).
Direct
information about the directions of the atomic moments in nanoparticles can be
obtained by Mossbauer spectroscopy. The Mossbauer spectra registered at 5 K in
a magnetic field of 4T applied parallel to the y-radiation of uniform
nanoparticles smaller than 5 nm prepared by laser pyrolysis (samples Laser1 and
Laser2) and precipitation in solution (solution), are shown in figure 16 [164].
The fitting of the spectra of samples Laser1 and Laser2 results in a nonzero
relative area of lines 2 and 5, which are very slightly reduced by the applied
field. The main effect was in the line broadening which affects the lines 1 and
6, suggesting that the directions of the atomic moments are highly disordered
for the laser samples due to a high degree of canting and spin frustration. In
contrast, the spectrum of a maghemite sample of similar particle size (between
3 and 5 nm), prepared by precipitation in the presence of oleic acid shows well
resolved A and B sites and can be fitted with two sextets. The area of lines 2
and 5 correspond to average canting angles of about 20° and 33°, much smaller
than the canting observed with samples Laser1 and Laser2. The effect of the
preparation method on the magnetic disorder is clearly demonstrated by the
spectra shown in figure 16 for sample Laser1, and especially for sample Laser2.
The fit of these spectra gives average hyperfine fields slightly smaller than
those of conventional microcrystalline maghemite particles (about 52 T), and
the hyperfine fields decrease with the particle crystallinity to about 48 T for
sample Laser2. This decrease in the average hyperfine fields is presumably also
due to the increase in the internal magnetic disorder [164].
4.2.
Interaction effects
The wide
variety of magnetic behaviour of nanostructured materials is complicated by
interparticle interactions, which limits
their possible application in biomedicine. For
sufficiently
dilute dispersions, interparticle interactions usually of a dipolar nature are
negligible and the crossover to the blocked state with decreasing temperature
depends only on the physical properties of the individual particles. However,
at higher densities (usually needed in practical applications) interparticle
interactions strongly affect the behaviour of the dispersion. In particular,
dipolar interactions between particles cause frustration of the moments, which
no longer align themselves precisely with the particles' easy axes at low T.
Rather, as the dispersion is cooled, collective, glassy behaviour results.
While this phenomenon has been studied extensively, most work to date has
focused on shifts of the blocking temperature, and the subtle question of
whether the cooperative freezing can be described as a true thermodynamic spin
glass transition [165,166].
We have
examined the effect of interaction on the magnetic properties of composites in
maghemite nanoparticles encapsulated in spherical silica particles that could
be used for biomedical applications [149,167]. In particular, we have analysed
the results using zero field cooling (ZFC) experiments and the standard
relation for the temperature variation of the reduced remanence (ratio between
the remanence magnetization and the saturation magnetization extrapolated at
0K, Mr(0)/Ms(0)) [168]. In the ZFC experiments we observed an increase of the
temperature at which the ZFC peak reaches its cusp with the increase in volume
packing fraction, which was associated with the increase in the interparticle
interactions. On the other hand, the fact that Mr(0)/Ms(0) values were in all
cases below 0.5 was explained from the effect of competition between
interparticle interactions and intraparticle anisotropy on the spin relaxation
process, which produces frustration [168-170].
5. Final remarks
The search
for new synthetic routes or the improvement of established ones which are able
to produce reliable magnetic nanoparticles with the correct characteristics of
improved tissular diffusion, colloidal stability and biocompatibility is in
continuous development. If we can gain sufficient understanding and control of
the biological reactions with the magnetic nanoparticle, we may be able to
control the rejection of nanomagnets by the human body. Ultimately, new
materials and understanding of their interaction with the body may lead to
better biocompatible nanomagnets.
For
example the application of magnetic liposomes (lipid vesicles, containing
submicron-sized magnetic nanoparticles in their structure either in the lipid
bilayer or in the aqueous compartment) as 'vehicles' for targeted drug delivery
appears to be a promising technique [171]. Liposomes can be used for
encapsulation of many biologically active substances, and can prolong their
therapeutic action by gradual release of the drug. Magnetic components of the
liposomes allow concentration of the liposomes in the desired area of the
patient's organs by magnetic forces, often augmented by magnetic agglomeration.
In this regard the work of Bulte and co-workers [172] is worthy of note. These
authors have developed magnetic liposomes derivatized with the hydrophilic
polymer polyethylene glycol (PEG) that may escape rapid uptake by cells of the
endothelial system.
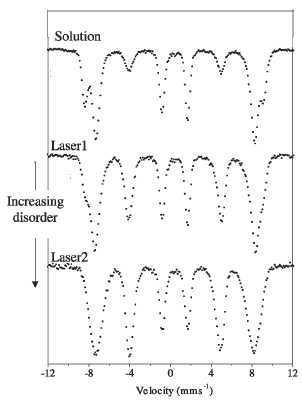
Figure 16. Mossbauer spectra at 5 K in the presence of
a magnetic field of 4 T applied parallel to the у-radiation for maghemite nanoparticles prepared by
different methods and with different degree of cationic disorder.
Drug and
gene delivery will continue to impact significantly on the practice of
biomedicine. Magnetic drug-targeting will undoubtedly dramatically improve the
therapeutic potential of many water-insoluble and unstable drugs. The
development of drugs able to target selected cells or organs within the body
will also deeply improve the benefits of some of the in vivo biomedical
applications of nano magnets. In this field the work of Bergemann and
co-workers [173] is worthy of note. These authors have succeeded in developing
novel iron oxide magnetic nanoparticles to which ion-exchange groups were
attached, thereby enabling simple and reversible binding of ligands. The
remarkable feature of ionically bound pharmaceutical drugs to the surface of
particulate drug delivery systems is that the active low molecular weight
substances can desorb from the carriers after a defined time span and hence diffuse
from the vascular wall into the tissue.
Acknowledgments
The
authors would like to thank K O' Grady for proof reading the manuscript. This
research was supported by CICYT under projects MAT2000-1504 and
MAT2002-04001-C02. The financial support from the regional government of Madrid
under project CAM 07N/0057/2002 is also gratefully acknowledged. PT
acknowledges the financial support from the Ramon y Cajal program.