Magnetic behavior of superparamagnetic Fe nanocrystals
confined inside submicron-sized
spherical silica particles
P. Tartaj,* T. Gonzalez-Carreno, O. Bomati-Miguel, and
С J. Sema
Instituto
de Ciencia de Materiales de Madrid, CSIC, Cantoblanco, 28049 Madrid, Spain
P.
Bonville
We have
studied the magnetic behavior of superparamagnetic Fe nanocrystals (4-7 nm in
diameter) dispersed in submicron-sized spherical silica particles (—150 nm in
diameter). Spherical composites that could be useful for biomedical applications
were prepared by an aerosol-assisted method. Mossbauer studies have allowed us
to determine that the magnetic response of the composites must be the result of
a competition between intraparticle anisotropy and interparticle dipolar
interactions. Evidence of an interacting superparamagnetic (ISP) regime that
is characterized by a HIMS scaling law of the reduced magnetization
isotherms instead the HIT scaling law of the ideal superparamagnetic regime has
been found in the composites. The ISP regime, as recently reported in similar
nanostructured systems, appears as an intermediate regime, separating the
high-temperature, conventional superparamagnetic regime from the
low-temperature, blocked-particle regime. We have also found that the
normalized values ofMs at room temperature are function of the Fe
metallic particle size. Finally, we have found that the magnetic anisotropy
constant of superparamagnetic Fe nanopar-ticles depend on the nature of their
coating shell.
I. INTRODUCTION
Nanocrystalline
magnetic materials often reveal unique properties that differ from their bulk
polycrystalline counterparts. Interest in this area comes partly from data
storage technology (e.g., hard disk drives1), partly from
biotechnology,2 and partly because nanomagnets provide a highly
controlled experimental system for studying fundamental phenomena in physics.3
A
particularly interesting physics occurs when nanomagnets are dispersed in a
matrix.4 The magnetic behavior of these systems can vary widely
depending on the size of the nanocrystalline particles as well as on the
packing fraction and the interaction between the nanomagnets and the matrixes.5"7
For an assembly of noninteracting fine particles the magnetic behavior is
understood on the basis of Neel arguments that led to the concept of
superparamagnetism, namely, the reversal of their magnetization through a thermally
activated process over the anisotropy barrier, even in the absence of an
externally applied field. For sufficiently dilute dispersions, interparticle
interactions are negligible and the crossover to the blocked state with
decreasing temperature depends only on the physical properties of the individual
particles. When the interparticle interactions become significant the behavior
of a magnetic moment is not only governed by its own intrinsic anisotropy
energy but also by the coupling with its neighbors. Although it has been
studied very intensively, it remains unclear how the magnetic interactions
affect the magnetic behavior of nanoscopic systems. Dipolar interactions cause
a frustration of the moments. In addition, there is a frustration resulting
from the competition between the interparticle dipolar and exchange terms and
the intraparticle anisotropy energy that requires the magnetization vector to
be aligned along specific axes in each particle.8
When the
interactions are strong enough, the particles may behave as a spin glass,9
although a true phase transition needs the combined effects of dipolar
interaction and anisotropy.10
A
knowledge of these fundamental properties is essential for the creative use of
nanomagnetic composites than can have tremendous potential and lead to improved
materials for applications in biology and medicine for the separation of
biochemical products and cells,11 magnetic resonance imaging
contrast enhancement,12 and a tissue specific release of therapeutic
agents.13 All of these applications depend on a magnetic material
with a modified surface that provides functionality to the composite. In this
way, the dispersion of nanomagnets in silica matrixes that can be easily
activated14 to provide functionality to the magnetic material seems
the ideal encapsulating material. In addition, the silica matrix greatly
enhances the wear and corrosion resistance of the magnetic nanoparticles, and
allows a fine tuning with temperature of the magnetic properties.15
Herein we
report a study of the magnetic behavior of superparamagnetic Fe metallic
nanocrystals (4-7 nm) confined inside submicron-sized spherical silica cages
(average size —150 nm in diameter) that could find applications in the
magnetically assisted chemical separation of biochemical products. Because this
application requires the preparation of stable liquid suspensions of magnetic
particles, the ideal mi-crostracture must consist of magnetic nanocrystals
dispersed in submicron-sized diamagnetic spherical particles that are expected
to have long sedimentation times in the absence of a magnetic field. The
composites were prepared by an aerosol-assisted method that was recently
reported to be adequate for the preparation of y-Fe2O3
nanocrystals confined within diamagnetic matrixes.16 Considering the
complexity of the system, before magnetic characterization, a crucial aspect we
have addressed is the detailed crystallochemical characterization of samples.
For example, it is known that some production methods guarantee nonmagnetic
oxide layers around each magnetic core (cluster, particle, or granule) while
others allow the cores to come into direct contact. This can make a profound
difference in the magnetic properties since the shell can exclude the direct
exchange interaction between particles so that the dipole force dominates at
all achievable volume fractions. If, on the other hand, the magnetic metallic
clusters are allowed to touch, exchange interactions could take place even at
low volume fractions.3 Further complication arises from the use of
methods, such as ball milling, that favor the presence of structurally
disordered grain boundaries showing a spin-glass-like behavior.17
Since the boundary spins are frozen in random directions the exchange
interaction cannot be transmitted across the interfaces.
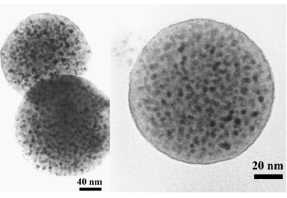
FIG. 1. Typical ТЕМ microstructures of Fe nanocrystals confined inside submicron sized
spherical silica particles. The dark spots correspond to the nanoparticles
containing the a-Fe metallic
II. EXPERIMENTAL PROCEDURE
Fe
nanocrystals confined inside submicron spherical silica particles were obtained
by an aerosol-assisted method. First, we prepared y-Fe2O3
nanocrystals confined in spherical silica cages. Details of the preparation of
the y-Fe2O3/silica composites can be found elsewhere.1516
Here it is sufficient to say that the powders generated after the aerosol
pyrolysis of a solution containing Fe(NO3)3 ■ 9H2O,
tetraethoxysilane and methanol were heated at different temperatures (800-1000
°C) in air to obtain composites containing y-Fe2O3
nanocrystals of different sizes. Fe nanocrystals confined inside submicron
spherical silica particles were obtained after reducing the y-Fe2O3/silica
composites to a-Fe in a H2 atmosphere at 500 °C for 10 h and cooled
to room temperature under the hydrogen atmosphere.
Phase
identification was carried out by x-ray diffraction (XRD) in a Philips PW1710
using CuKa radiation. Fe crystallite sizes were estimated from the full width
at half maximum of the reflection (110) of a-Fe by using the Scherrer
equation. Particle size and shape of the samples were examined by transmission
electron microscopy (ТЕМ, Jeol 2000 FX).
57Fe Mossbauer absorption spectroscopy was used to characterize the
samples. The spectra were recorded with a maximum velocity of 10mms ~l
at different temperatures with a 57Co:Rh source. Magnetic properties
of the samples were recorded in a vibrating sample magnetometer (MLVSM9 MagLab
9 T, Oxford Instrument). The saturation magnetization (Ms) and
coercivity field values (Hc) were obtained from the hysteresis loops
registered up to a field of 5T. Ms values were obtained from the law
of approach to saturation.
TABLE I. Total amount of Fe present in
samples and phase composition in wt% as determined by Mossbauer spectroscopy.
Uncertainties in the weight composition were about 5%.
|
Sample |
F1 |
F2 |
F3 |
F4 |
|
Fe/(Fe + SiO2) (wt%) |
15 |
15 |
15 |
25 |
|
Fe |
6 |
4 |
3 |
12 |
|
Fe2SiO4 |
8 |
11 |
13 |
13 |
|
Fe2O3 |
5 |
5 |
5 |
7 |
|
SiO2 |
81 |
80 |
79 |
68 |
III. RESULTS AND DISCUSSION
A. Crystallochemical characteristics of samples
After
reduction (see Sec. II for details) the samples consisted of iron nanocrystals
(XRD only showed diffraction peaks due to a-Fe) distributed inside the
submicron sized spherical silica particles (Fig. 1). In order to study size and
interparticle effects, composites with different Fe particle sizes and
Fe/silica compositions were prepared. A summary of the main physical
characteristics of samples can be found in Tables I and II. It is worthy of
note that composites with a lower load of magnetic material were not prepared
because, as we will see below, the content in a-Fe was very low. On the other
hand, a higher load of magnetic material led to composites in which some
uncontrolled interparticle sintering was observed.
TABLE
II. a-Fe crystallite size (DXR) and values of Ms at RT
normalized to the a-Fe content in samples. Values of the median blocking
temperature (TB) and the standard deviation cry obtained
from the fit of the decay of the reduced remanence. The uncertainties are
about 1-2 К for (TB) and 0.1 for ay.
The values of the mean blocking temperature Tm and the anisotropy
constant KR derived from Tm are also shown. The
uncertainties in Tm and KR were obtained from the
uncertainties in (TB), cry, and the particle diameter.
Finally, the values of the magnetic anisotropy constant KLAS determined
from the law of approach to saturation and the values of H, at 5 К are also shown.
|
Sample |
F1 |
F2 |
F3 |
F4 |
|
DXR (nm) |
6.9 (0.5) |
5.2 (0.5) |
4.4 (0.5) |
7.3 (0.5) |
|
Ms (emu/g Fe) |
205 |
180 |
160 |
210 |
|
(TB) (K) |
61 |
29 |
19 |
66 |
|
o-y |
0.95 |
0.95 |
1.00 |
1.00 |
|
Tm (K) |
25(5) |
13(3) |
7(2) |
24(6) |
|
KR(XW4 Jm"3) |
5(2) |
6(3) |
5 (4) |
4(2) |
|
i:LAS(X104 Jm-3) |
5.4 |
6.9 |
7.7 |
5.3 |
|
Hc (Oe) |
450 |
390 |
330 |
490 |
XRD
cannot discard the presence of other phases such as the iron oxide spinel
normally formed during a-Fe corrosion processes.1920 This phase
normally appears in the form of nanocrystals of about 2 nm in size (with a
grain size lower than ~2 nm, diffraction effects are diffuse and close to the
background noise) that combined with their low content could be the reason for
their absence in the XRD patterns. Alternatively, because the reduction is
carried out inside a silica matrix, we cannot disregard the presence of an
outer shell of iron (II) silicate surrounding the inner Fe metallic core.
Therefore, we registered the Mossbauer spectra of the samples to determine the
possible presence of any other iron-containing phase apart of a-Fe.
Figure 2
shows the Mossbauer spectra of sample F1 (chosen as representative) registered
at different temperatures. The other samples showed similar spectra only with
differences in the relative amount of phases. It is worthy of note that we
have assumed similar effective Debye-Waller factors for all the different
phases present on the samples as previously assumed by Hadjipanayis and
co-workers when working on a similar system.2122 Recently Kuhn et
al. confirmed the validity of this assumption also for a similar system.19
At room temperature (RT) the spectrum consists of a single line with an isomer
shift of 0.0 mm s : that is due to the presence of a fraction of
superparamagnetic a-Fe particles, and a sextet with an isomer shift of 0.0 mm
s~:, a quadrapole splitting of 0.0 mms"1, and a
hyperfine field of 32.5 T, which corresponds to the fraction of a-Fe particles
that are blocked at RT. The fraction of a-Fe particles that remains superparamagnetic
is about 30% for this sample. Supporting the presence of superparamagnetic
a-Fe at RT, samples F2 and F3, that according to XRD contain Fe nanocrystals
with a mean size smaller than sample F1 (5.2 nm for sample F2 and 4.4 nm for
sample F3 versus 6.9 nm for sample Fl), have a larger fraction of superparamagnetic
a-Fe particles at RT (60% for sample F2 and 70% for sample F3). Moreover, the
fraction of a-Fe particles that remains superparamagnetic in sample F4 that
have a similar crystallite size was similar (30%).
The
Mossbauer spectrum at RT also displays the presence of a doublet with an isomer
shift of 1.04 mm s : and a quadrapole splitting of 1.59 nuns"1
that is characteristic of high-spin Fe (II) cations in octahedral coordination.
It arises most likely from the nanoparticle/silica interface, where Fe (II)
cations exist in an environment similar to that of Fe2Si04.22>23
In accordance with this interpretation, the relative content of this phase
increase with the decrease of the iron core crystallite size (smaller particle
sizes involve larger surface areas and therefore larger contact area). Finally,
the spectrum displays a non-resolved sextet (mainly consisting of a central
broad quadrupolar doublet) with an isomer shift of 0.4 mms4 that
could be associated with the presence of ferric oxide nanoparticles. The fact
that the relative content of this phase was independent of particle size, i.e.,
surface area (see, in Tables I and II, samples F1, F2, and F3) seems to suggest
that its presence is due to the incomplete reduction of the samples rather to the
presence of an iron oxide corrosion layer formed during passivation processes.
In order
to further elucidate the nature of the phases present on the samples, we
registered the spectra of sample F1 at lower temperatures (Fig. 2). At 150 K,
the spectrum displays a sextet associated with a-Fe. However, no single line
associated with superparamagnetic Fe nanocrystals is detected, which means that
all Fe nanocrystals are blocked (according to Mossbauer) at this temperature.
The spectrum also displays a sextet with an isomer shift of 0.37 mms"1
that can only be fitted to a distribution of hyperfine fields. This
signal corresponds to the ferric oxide nanoparticles. Finally, the spectrum
displays the doublet associated with Fe (II) cations in an environment similar
to that of Fe2Si04. At 100 K, the spectrum is similar to
that observed at 150 K; the only difference is that the sextet associated with
ferric oxide nanoparticles appears better resolved.
At 50 K,
the most significant result is that we observe a decrease in the doublet
associated with Fe2Si04 from 30 to 20%. This decrease is
more evident at 4.2 K, where the signal associated withFe2Si04
represents only а 10%, while the one associated with
a-Fe increases from 36% to 50% and the one associated with ferric oxide
increases from 32% to 40%.
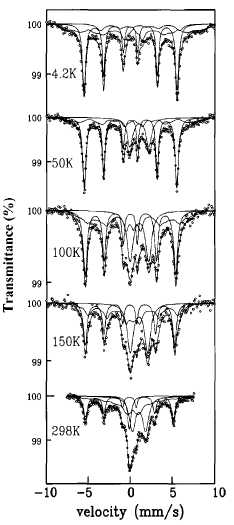
FIG. 2. Mossbauer spectra for sample F1.
Fe2Si04
is antiferromagnetic and has an associated Neel temperature of 65 K.24
Thus, at temperatures below 65 К we should
observe the signal associated with the magnetic hy-perfine splitting. Moreover,
because of the small particle size, we must have a significant fraction of this
phase that remains superparamagnetic especially at 50 K. However, the magnetic
hyperfine fields of Fe (II) components are very sensitive to local distortions
caused by, for example, defects, because of the orbital contribution to the
magnetic hyperfine field. Therefore, if there are variations of the local
environment of the Fe (II) cations, the Fe (II) components may be smeared out
such they are not visible as separate components,19 and this could
be the reason we can only observe the fraction of the Fe2Si04
that remains superparamagnetic. Finally, it is worthy of note that the value
of the hyperfine field obtained for the a-Fe metallic core at 4.2 К (34.0 T) is similar to that obtained for pure a-Fe
(33.9 T),25 which excludes the possibility of having silicon atoms
forming a Fe-Si alloy. A summary of the phase composition of samples obtained
from the Mossbauer analyses can be found in Table I.
The temperature
variation of the spectral features described above and the hyperfine
parameters of the spectral components derived from the fit suggest two possible
scenarios to represent the iron-containing nanoparticles encapsulated in the
spherical silica particles: (1) The nanoparticles consist of an inner central
core of the iron oxide that remains unreduced, surrounded by an outer central
core of a-Fe that is encapsulated in an outer shell of Fe2Si04;
(2) we have nanoparticles consisting of an a-Fe core that is encapsulated in a
Fe2Si04 shell, and separately we have iron oxide nanoparticles
that remain unreduced. These iron oxide nanoparticles are most likely located
close to the center of the bigger spherical silica particles and thus are
difficult to reduce. It is worth mentioning that more severe thermal treatments
to fully accomplish the reduction of the composites have not been studied
because they drive to uncontrolled interparticle sintering. Independently of
which of the two scenarios better represents the distribution of the
iron-containing phases, it is clear that in our system we can exclude a-Fe
metallic cores from coming into contact, and thus direct exchange interactions
can be discarded. In this way, their magnetic response must be the result of a
competition between intraparticle an-isotropy and interparticle dipolar
interactions.
B. Magnetic behavior of samples
In data
storage applications, the particles must have a stable, switchable magnetic
state to represent bits of information, a state that cannot be affected by
temperature fluctuations, for example. However, for biomedical applications
the use of particles that present a superparamagnetic behavior at room
temperature (no remanence along with a rapidly changing magnetic state) is
preferred.26 In order to check for superparamagnetic behavior, we
registered the hysteresis loop at RT in our samples (Fig. 3). We can clearly
see that the particles are superparamagnetic (i.e., the value of Hc
is zero).27 Samples containing Fe nanocrystals of sizes larger than
those here presented (s=8 run) did not show a super-paramagnetic behavior at RT
and thus were not studied.
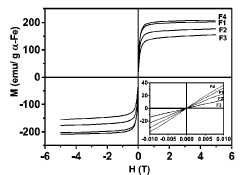
FIG. 3. Hysteresis loops of samples at
RT. The inside plot is a magnification of M-H at low magnetic field (from —0.01
to 0.01 T) to show the superparamagnetic behavior of the composites (zero
coercivity field). Values of M were normalized to the a-Fe content.
For iron
nanocrystals between 4 and 7 nm we expect the Ms values to be
similar to those of bulk (220 emu/g) at temperatures of about 5 K,17>28
and indeed this was the case when the a-Fe content, obtained by quantitative
analyses of the Mossbauer spectra at RT and 150 К to assure that all the Fe2Si04 is visible, was
used to determine the normalized Ms values for all the samples. In
addition, this result seems to suggest that the estimation of the phase content
on samples by Mossbauer was reliable. On the other hand, these Ms values
at RT (Table II) were, in all cases, lower than that of bulk Fe, which reflects
the small particle size of the Fe nanocrystals. Theoretical calculations on
ferromagnetic clusters carried out by Hendriksen etal29 have shown
that a finite particle size can cause a sizable deviation from the normal Bloch
T312 law and that the Curie temperature can be reduced for the
smallest particles.30 Supporting this interpretation the normalized
Ms values at RT increased as the crystallite size increased (in
fact for the samples with a crystallite size about 7 nm were closed to the bulk
value) and for the samples with different composition but similar crystallite
size were similar (Table II). It is worthy of note that the values of Ms
remained almost invariant after six months, i.e., the samples were stable,
which is probably due to the combined effect of the silica matrix and the iron
(II) silicate protective layer.
As
mentioned above, a possible use of these particles is in the biomedicine field.
Therefore, it could be of interest to check for the presence of dipolar
interactions between the superparamagnetic nanomagnets confined inside the
spherical silica particles to better predict the magnetic response of these
composites. A comprehensive analysis of the possible presence of dipolar
interactions was carried out with the help of a mean-field model, recently
proposed by Allia et al.21 and later on verified by Binn et al3
The use of this model could allow us to estimate dipolar interactions at a
temperature region, in which the so-called interacting superparamagnetic regime
describes the behavior of interacting nanomagnets. In particular, in this
region dipolar interactions can be characterized by a parameter T*, appearing
in the denominator of a modified Langevin function analogous to the Curie-Weiss
law:
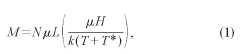
where N
is the number of moments per unit volume, /л is the particle moment, L is the Langevin function, к is the Boltz-mann constant, H is the applied magnetic
field, and T is the temperature. The parameter T* is proportional to the
dipolar energy and can be obtained from the following expression:31
![]()
where a
is a proportionality constant deriving from the sum of all dipolar energy
contributions,32 N is the number of moments per unit volume and к is the Boltzmann constant, a and N can obtained from
the low-field susceptibility data, x, using the expression
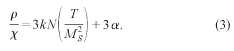
We have
assumed a log-normal distribution to estimate p in Eq. (3). The use of this
type of distribution to describe systems containing magnetic nanocrystals has
been previously shown to be adequate.33 If our system follows the
ISP regime in a particular range of temperatures, we can expect a linear
dependence of the quantity plx on the ratio T/M2S and we
can easily obtain the values of a and N (hence T*). The linear dependence of
the quantity plx on the ratio T/M2S is clearly displayed
in Fig. 4 for all samples.34 This result supports a picture of an
interacting superparamagnetic regime as recently reported in similar
nanostructured systems. This ISP regime appears as an intermediate regime,
separating the high-temperature, conventional superparamagnetic regime from the
low temperature, blocked-particle regime. The values of T* at 300 К obtained from the best linear fits were lower for
sample F1 (360 K) than for sample F4 (450 K), which correlates well with the
lower Fe content of sample F1. In the composites having the smaller Fe
nanoparticle sizes and the lower volume fractions (F2 and F3), the dipolar
interactions were weaker, and thus at RT the values of T* for these two samples
were close to zero, i.e., the composites behave as an ideal superparamagnet.
However, at lower temperatures we can expect a transition from an ideal
superparamagnetic regime to an interacting superparamagnetic regime, as
reported in similar nanostructured systems.331 Further confirmation
of the occurrence of interparticle interactions in the composites were obtained
after analyzing the low temperature variation of the coercivity. In the
absence of interactions, the coercivity should follow the well-known expression Hc(T)=Hc(0)[l-(T/TB)m]35
In our samples we do not observe this
dependence (Fig. 5), which is in accordance with the occurrence of
interparticle interactions in all the characterized composites.36
El-Hilo
et al37 found that the blocking temperatures, obtained from
measurements of isothermal remanence for fer-rofluids with different
concentrations of 8-nm iron oxide particles, were nearly identical, and they
concluded that the decay of remanence was not sensitive to interaction effects.
Moreover, Morap et al.,9 also working with y-Fe2O3
nanocrystals of about 8 nm coated and uncoated with a layer of oleic acid,
showed similar results. Only for the case of an uncoated powder pressed at
about 1300 MPa did these authors note a slight increase in the blocking
temperature that they associated with interaction effects. In our samples, according
to Mossbauer, the iron nanocrystals are coated by an iron (II) silicate shell.
Moreover, the a-Fe volume packing fraction is very low, and therefore we could
expect that the blocking temperatures estimated from the variation of the
reduced remanence must be insensitive to interaction effects. Figure 6 shows
the reduced remanence data as a function of temperature for all the samples. We
have analyzed the results using the standard relation for the temperature
variation of the reduced remanence (normalized to the measured saturation
magnetization), which allows one, among other things, to obtain quantitative
information about the mean blocking temperature. The relation is given by
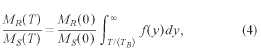
where (TB)
is the median blocking temperature, у = TBI(TB) is the reduced blocking temperature,
and MR(0)/Ms(0) is the reduced remanence at 0 K. The
distribution/^) of reduced blocking temperatures is assumed to be a log-normal
function:
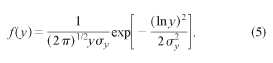
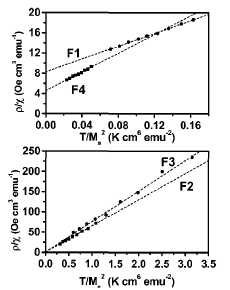
FIG. 4. Variation of the quantity plx on the ratio
TIM\ for all samples in a temperature range from 200 to 400 K. The solid line
represents the best linear fit of the data using Eq. (3).
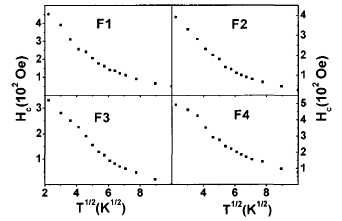
FIG. 5. Variation of Hc with T112
for all the samples. The nonlinear behavior clearly discards the j thermal
dependence of the coercivity.
The best
fits with Eq. (4) to the data are shown by the lines in Fig. 6, and the values
of (TB) and the standard deviation иy are given in Table II. From the
values of (TB) and the standard deviation ay, we can
obtain the mean value of the blocking temperature using the expression Tm =
(Гв)ехр(-о^). As expected, the values of Tm (Table
II) increased with the increase of particle size, and, for samples with similar
particle sizes but different volume packing fractions, the values of Tm
remained unchanged (as predicted the decay of remanence is mainly determined by
anisotropy). From the values of Tm and particle size obtained by
XRD, we can make an estimation of the magnetic anisotropy constant К using the expression KV= kBTBln(тт/т0), where V is the particle volume, kB is
the Boltzmann constant, т0 is the characteristic time, and rm is the
measuring time. Considering the typical values for т0 and assuming rm~
100 s for a measurement carried out in a vibrating sample magnetometer,2837
the values of the magnetic anisotropy constant (Table II) are very similar for
all samples and close to the value reported for bulk Fe (4.8X 104
Jm~3).18 However, the experimental uncertainties
preclude us to discard small variations in the values of the anisotropy
constant. Thus, attempts were made to determine more precisely the anisotropy
constant. Particularly, we determined the value of К from the magnetization data at 5 К using the law of approach to saturation:18
![]()
where Ms
is the saturation magnetization, Xf is the high-field susceptibility, and В is function of Ms and K, and is given by
the following expression:38
![]()
The
obtained values (Table II) are close to the bulk value for samples F1 and F4
while an increase is observed in samples F2 and F3.39 In any case,
the enhancements of the anisotropy value observed in our samples with respect
to the bulk were smaller to that reported in iron nanoparticles that consisted
of a a-Fe metallic core and an iron oxide passivation shell21 (5X105
Jm~3, ~1 order of magnitude). This increase was associated with the
interaction between the iron oxide passivation shell and the Fe metallic core.
In our system, as determined by Mossbauer spectroscopy, apart from the
presence of ferric oxide, we have the metallic Fe surrounded by an appreciable
amount of an iron (II) silicate protective layer. Therefore, we could expect
the interaction between the metallic core and the surface of these two systems
to be different. Particularly, it seems that in our system this interaction is
weaker. Alternatively, we can speculate that the observed enhancement in the
anisotropy constant of the Fe nanoparticles coated by an iron oxide passivation
layer (normally y-Fe2O3 or Fe3O4)
(Ref. 19) could have some contribution from the iron oxide itself. In fact, enhancements
of two orders of magnitude (from 4.8 X 103 Jm~3 of bulk
maghemite to —5X105 Jm~3) have been observed in y-Fe2O3
nanoparticles, and have been associated with the existence of a magnetically
disordered surface layer.40 41
A further
confirmation of the veracity of the anisotropy constant values was obtained
from the values of Hc at 5 К (Table II). For example, for an assembly of noninteracting randomly
oriented single-domain cubic particles the value of coercivity can be
determined by the expression Hc = 0.64K/Ms (200-300 Oe
for the range of anisotropy constants determined by the law of approach to
saturation), while foruniaxial particles Hc = 0.96K/Ms
(300-450 Oe for the range of anisotropy constants determined by the law of
approach to saturation). Variations with respect to these theoretical values
can be associated for example with interpar-ticle interactions or interactions
between the Fe nanoparticles and the matrix.42 Finally, it is worth
mentioning the decrease in Hc values with decreasing particle size,
which could reflect the presence in composites having smaller Fe nanoparticles
of a fraction of composites that remains unblocked at 5 K. In fact, as observed
in the variation of the reduced isothermal remanence with temperature (Fig. 6)
the values at 5 К for samples F2 and F3 were
smaller. Alternatively, we cannot discard some slight contribution from
anisotropy shape in the samples having bigger sizes.
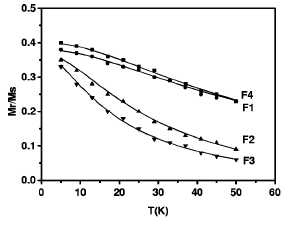
FIG. 6. Variation of the reduced isothermal remanence with temperature for all the samples. The solid lines represent the best fit of the data with a standard decay-of-remanence model.
IV. SUMMARY AND CONCLUSIONS
Mossbauer
studies have allowed us to determine that the magnetic response of iron
nanoparticles dispersed in submicron-sized silica particles must be the result
of a competition between intraparticle anisotropy and interparticle dipolar
interactions. We have also found that in our system a superparamagnetic
behavior at RT is found for Fe nanocrys-tals below about 8 nm in diameter.
However, studies of the thermal dependence of the magnetization have shown evidence
of an interacting superparamagnetic regime in our samples, as recently observed
in similar nanostractured systems. We have also found a dependence of the
values of Ms with the Fe particle size at RT. Finally, we have
determined
that the
enhancements of the anisotropy values observed in our samples with respect to
the bulk were smaller that reported in iron nanoparticles that only consisted
of a Fe metallic core and an iron oxide passivation shell. The reason,
therefore, for this discrepancy could be either associated with the presence in
our samples of an iron (II) silicate shell coating the Fe metallic core or to
the lower relative content of a ferrimagnetic iron oxide layer.
ACKNOWLEDGMENTS
Financial
support from CICYT (MAT2002-04001-C02) is gratefully acknowledged. P. T. thanks
the financial support from the Ramon у Cajal program.