HEAT AND MASS TRANSFER PHENOMENA
E. Blums
Institute of Physics, University of Latvia, LV-2169, Salasplis, Latvia.
Energy conversation.
Most of devices employing magnetic fluids are working
under the non-isothermal conditions. Temperature gradients can be applied
externally, or are generated by adiabatic processes (under the effect of a
compressions and a magnetization of the fluid) or due to viscous dissipation of
energy in flows under the shear stress. The compressibility caused temperature
changes in liquids as well the magnetic demagnetization effects, are relatively
small [1]. Only close to the Curie temperature, the adiabatic magnetization can
cause a considerable change in fluid temperature. This phenomenon at early
stage of the magnetic fluid research was proposed to use for a thermomagnetic
energy conversion [2]. In colloids containing magnetically «hard» particles, the
fluid magnetoviscosity can significantly effect the thermal dissipation of
flow energy. The specific heat of colloid and its density can be calculated
under the assumption of additivity employing the know coefficients of particles
and that of carrier liquid. The thermal conductivity of colloids of spherical
particles follows to a classical dependence of effective medium [3]. Only for
dispersions of nonspherical particles or if the aggregate formation takes
place, the thermal conductivity depends on magnetic field. In the presence of
an orthogonal field B ^ÑT the thermal conductivity decreases and opposite, in
a longitudinal field B II ÑT it increases
[1].
Thermomagnetic convection
To analyze the convective processes, the energy conservation equation should be considered together with the equation of a fluid motion, which contains a new term of magnetostatic force. Considering the problem of thermoconvective stability, in Ref [4] it is sown that without applying external temperature gradients the ferrofluid with respect to adiabatic compression and magnetization is always stable. In the presence of external temperature gradients the adiabatic terms usually can be neglected. In such approximation the Raleigh number contains two additive terms reflecting the thermogravitational and the thermomagnetic buoyancy forces. An interesting situation appears when the nonisothermal ferrofluid layer is subjected by a homogeneous magnetic field. Thanks to the pyromagnetic properties of the fluid, a gradient of internal magnetic field in the layer appears. As a result, the Raleigh number becomes a square dependent on the temperature gradient. It means that the thermoconvective instability can develop independently of the gradient and the acceleration of gravity. The general conclusions of the thermoconvective stability theories (the pioneering one, obviously, is Ref. [5]) are confirmed experimentally (see, for example Ref. [6]). If an external gradient of magnetic field is applied non-parallel to the ÑT, the intensity of magnetic convection can significantly exceed the thermogravitational one. It means that magnetic control of the heat transfer in ferrofluids is an interesting problem of applications.
Mass Transfer
Colloidal
particles in magnetic fluids obey an intensive Brownian motion. Therefore, the
mass transfer can be considered similar to that of molecular liquids. The
diffusions coefficient of nanoparticles determined by relation of
nanoparticles determined by relation D =
kT / f (f is
the hydrodynamic drag force) is several order of magnitude less than that for
molecules. The mass flux contains a new barodifusion term: besides the
gravitational sedimentation it is necessary to take into account also the
magnetic sedimentation. The particle transfer under the action of the uniform
gravitation force always causes an increase of the fluid convective stability.
The magnetic force usually is not homogeneous. As a result, the magnetic
sedimentation forms concentration gradients, which always are oriented opposite
to the driving force. Thus, the magnetic stratification of fluids is unstable.
Reaching the critical solutal raylegh number value, the onset of a specific
diffusion-magnetic convection should be observed even in isothermal colloid
[7]. Theis effects is confirmed experimentally [8,9].
Heat and Mass Transfer Problems
Recent experiments refer to high thermodiffusion coefficients of nanopartricles in ferrocolloids [10 – 12]. Surfacted ferrite particles are transferred toward decreasing temperatures (positive Soret coefficients) [10], but electrically stabilized particles in ionic colloids usually have negative Soret coefficients [11, 12]. The effect is so strong, that in the thermodiffusion column experiments almost complete seperation of ferroparticles from the carrier liquid can be achieved [13]. If the uniform magnetic field is applied, the internal field gradients can additionally induce a specific thermomagnetophoretic transfer of feroparticles [1]. This so-called «magnetic Soret effect» and its anisotropy recently have been observed experimentally [14]. The thermophoretic redistribution of particle concentration causes an additional gradient of internal magnetic field. As a result, also the mass diffusion coefficient becomes field dependent and anisotropic [15]. The very high Soret coefficient of ferroparticles is not only an important practical problem for ferrofluid applications but brings up new problems of Soret-driven solutal convection [13] and that of the stability of double diffusive magnetic convection [16].
References:
1. Blums E., Cebers M., Maiorov M. M.. Magnetic Fluids (Walter de Gruyter, Berlin, New York, 1997).
2. Rosenszweig R. E. Ferrohydrodynamics (Cambridge University Press, Cambridge, 1985).
3. Tareev B. M. // Coll. Journ., 6 (1940) 545 (in Russian).
4. Shliomis M. I. // Fluid Dyn. 6 (1973) 957.
5. Finlayson B. A. // J. Fluid Mech. 40 (1970) 753.
6. Schwab L. Ph. D. Thesis, Iniversitat Munchen, 1989.
7. Chukhrov A. Yu. Magnetohydrodinamics, 22 (1986) 254.
8. Blums E., Chukhrov A. Yu., Rimsa A. // Int. J. Heat and Mass Transfer 30 (1987) 1607.
9. Odenbach S. Ph. D. Thesis, Universitat Munchen, 1993.
10. Blums E., Mezulis A., Maiorov M., Kronkalns G. // J. Magn. Magn. Mat. 169 (1997) 220.
11. Lenglet J., Ph. D. Thesis, Universitaty Paris 7, 1996.
12. Mezulis A., Ph. D. Thesis, Universitaty Paris 7, 1999.
13. Volker T., Blums E., Odenbach S. // Magnetohydrodynamics 36 (2001) (to be published).
14. Blums E., Odenbach S., Mezulis A., Maiorov M. // Phys. Fluid 10 (1998) 2155.
15. Bacri J. C., Cebers A., Bourdon A., Demouchy A., Heegard G., Kashevskiy B. M., Perzinskiy R. // Phys. Rev. E 52 (1995) 3936.
16. Shliomis M. I., Souhar M. // Europhys. Letter, 49 (2000) 55.
THERMODIFFUSION IN MAGNETIC FLUIDS
T. Volker 1, E. Blums 2, S. Odenbach 1.
1.
ZARM, Universitat Bremen, Am
Fallturm, 28359 Bremen.
2.
Institute of Physics, University of
Riga, LV-2156 Salaspils.
Motivation and theoretical background.
Transport properties in magnetic colloids play an
important role concerning the problem of long-term stability of ferrofluids. An
attempt to measure particle mobility in the fluid was made by E. Blums in 1983
[1]. The investigations based on non-stationary particle separation
measurements using a thermodiffusion column. It consist of a vertical flat
channel – a small gap between two plates held at different temperatures T1
and T2 – and two connected separation chambers. A concentration
profile in the gap develops caused by thermal diffusive particle transfer along
the temperature gradient. This leads in combination with convective transport
in the gap to an increase of a particle concentration in the lower and an
analogical decrease in the upper chamber.
At the moment two analytical calculation for the Soret
coefficient describing the separation problem exist: One is valid for the
initial part of separation and the other for the steady concentration
difference. Up to now the corresponding Soret coefficient is evaluated from the
unsteady part of separation curves by using an empirical analysis [2] because
of limited experimental time and experimental problems at the initial part of
separation.
To get closer insight into these problems we have
designed two independent experimental setups with indentical thermodiffdusion
columns and measuring devices, one for long-time experiments and the other for
short-time experiments.
The present paper deals with experimental results of
separation dynamics in the thermodiffusion column in which we reach the regime
of steady concentration difference. In this state separation the concentration
difference follows [3].
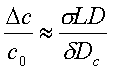 (1)
(1)
Here Dj/j0 is the standardized concentration
difference between the upper and lower chamber, s is the nondimensional particle separation parameter,
L is the height of the separation channel, D is the Brownian diffusions
parameter, d is the width of the gap and DC
is the convective diffusion coefficient. For small values of S (S = k GrC
/ GrT , GrC is the concentration Grasshof number, GrT
the thermal Grasshof number and k is a non-dimensional thermodiffusion
parameter, which is proportional to the Soret coefficient) the concentration
difference in the column chambers is given in the asymptotic regime by [4].
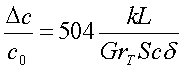 (2)
(2)
where Sc is the Schmidt number.
The time to reach a steady concentration difference is
in the order of weeks. If concentration increases (S>>1), a higher
separation difference is reachable but the experimental time increases rapidly
as well.
In the present paper we compare the Soret coefficient for magnetite particles found from the steady and unsteady part of the separation process.
Experimental procedure and setup.
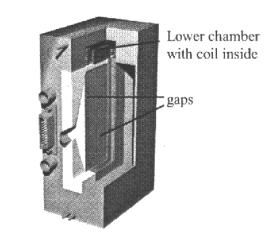 Figure 1.
Thermodiffusion column.
Figure 1.
Thermodiffusion column.
The
separation measurements are performed by using a vertical flat column (figure
1) of width d = 0,5 mm and height L = 90 mm. The heated and cooled walls
are held by thermostats at constant temperatures T1 = 20°C and T2
= 30°C. Particle concentration in both chambers is determined by
measuring the resonance frequency of a LC-oscillator. Therefore the coils
inside the two chambers are connected with two independent oscillators. The
inductance of the coils increases linear with volume concentration of magnetic
particles, leading to a decrease of the resonance frequency of the connected
oscillator.
Results
and discussion.
Figure 2 shows the separation curve in the initial part measured in zero field with DT = 10 K (T1 = 20°C, T2 = 30°C, T0 = 25°C).
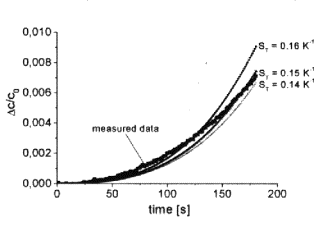
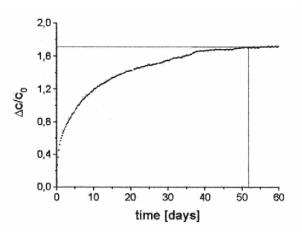
Figure 2. Initial part of the thermodiffusion process. Figure 3. Steady state regime.
The plotted thin lines are the curves
from the analytical model for different values of the Soret coefficient [5].
The measured separation curve shows the Dc / c0 » t 2,5
behavior for times t £ 200
s. The small difference to the
calculated curves reflects not only the measurements error but also the
uncertainty of D0. Expressions used for the calculation based on the
assumption of monodispersity of particles and negligibility of particle
interaction. The comparaison between calculated and experimentally found
curves gives a value for the Soret coefficient of ST = + 0,15 ± 0,02.
Figure 3 represent the long term
development of the separation process up to saturation which is reached after
approximately 51 days [5]. This investigation has been also performed for H =
0, using a temperature difference of 8 K (T1 = 29°C, T2 = 37°C).
The separation level Dc / c0 = 1,72 is extremely high; from the initial
concentration c0 = 0,017 the concentration in the lower chamber is c1
= 0,0326 and cU = 0,0012 in the upper one. Between the initial and
saturation part we found time ranges with with linear (250 s £ t ³ 2000 s) and square root time dependence of Dc (2100 s £ t ³ 10 h), the later one being predicted theoretically.
References:
1.
E. Blums, G. Kronkalns, R. Ozols //
J. Magn. Magn. Mat. 39 (1983).
2.
E. Blums, S. Odenbach, A. Mezulis,
M. Maiorov // Phys. Fluids 9 – 10
(1998) 2155 – 2163.
3.
E. Blums, A. Mezulis, M. Maiorov,
G. Kronkalns // J. Magn. Magn. Mat. 169
(1997), 220 – 228.
4.
E. Blums // J. Magn. Magn. Mat. 149
(1995) 111 – 115.
5.
T. Volker, E. Blums, S. Odenbach //
Magnetic hydrodynamics, V. 36, № 2 (2000).
TRANSPORT
PROPERTIES OF AN
IONIC MAGNETIC COLLOID:
EXPERIMENTAL STUDY OF INCREASING
THE IONIC STRENGHT
A. Mezulis, M. Mairov, E. Blums.
Institute of Physics, University of Riga, LV-2156 Salaspils.
Introduction.
Magnetic colloids (ferrofluids) are colloidal dispersions of magnetic particles of typical size about 10 nm in a liquid carrier. Two ways to eliminate the aggregation of particles are known: a steric hindrance provided by a surfactant coating of particles (surfactes ferrofluids), or ensuring the electrostatic repulsion by charging the particles (ionic ferrofluids).
Main transport properties of magnetic colloids are
translational mass diffusion coefficient DM and thermal diffusions
(Soret) coefficient ST, determining in a binary mixture the particle
volume fraction flux j quantitatively:
j = – DM ( Ñ j + ST j (1 –
j)Ñ T ), (1)
where j is the particle volume concentration and T is the temperature.
Transport coefficient DM and ST have been investigated of late years by different experimental methods [1, 2, 3]. Development of exact theoretical models of the Soret effect in magnetic colloids demands more investigations in this field. Due to this, the dependence of transport properties of an ionic ferrofluid on ionic strength may be of great intrerest, being experimentally studied in present work.
Ionic ferrofluid and the effect of phase separation.
An ionic ferrofluid sample, used in these experiments,
consist of positively charged g-Fe2O3 particles in acid aqueous medium,
neutrality of which is reached by adding NO3 – counterions. Magnetogranulometric analysis
indicates the standard deviation s = 0,38 for a lognormal particle size distributions,
the average diameter of hard particles is found as d0 = 10,5 nm. Measurements od density indicate j0 = 6,3 %.
The total ionic strength I of a solution:
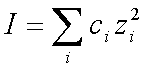 (2)
(2)
where
ci is the concentration
and zi the valence of the
i-sort of presented ions, is low in a pure ferrofluid sample, but it can be
increased very many times by dissolving a salt, e. g. NaCl. However, the effect
of phase separation in ionic ferrofluids by increasing the ionic strength is
well known [4]. This effect appears in the form of separation into two liquid
phases at reaching the threshold ionic
strength of the counterions I0: the formation of settling
spherical droplets (in diameter of some micrometers) of concentrated phase
occurs in the more diluted one. Experiments prove that I0 for a given sample is rather independent of initial
volume concentration j0, whereas it is strongly dependent on particle size distribution [4].
Authors give the lowest threshold ionic strength of the counterions of 0,2 –
0,25 mol / l.
Experimental section.
Experiments of measuring DM and ST as functions of
ionic strength have been performed by means of a grid setup, described
particularly in Ref. [3]. The salt is added to the magnetic colloid by
dissolving various doses of a 0,5 mol / l NaCl solution. The best dose is
chosen to reach the ionic strength of the counterions 0,14 mol / l (in fact,
only of Cl – ions, as I (NO3
– ) << I (Cl – )),
being safely below the threshold valure I0. Two salt dosing series have been
carried out, 4 – 6 measurements of DM
and of ST with each dose
were performed.
Results and discussion.
Main results of performed experimental work are collected in Figures 1, 2.
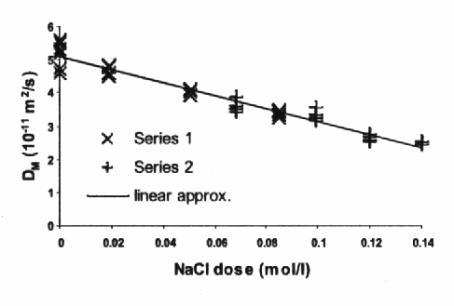
Figure 1. Measured translational mass diffusion
coefficient DM with adding NaCl.
In the first degree of approximation, obtained dependence in Figure 1 is linear. Concerning Einstein-Stokes formula:
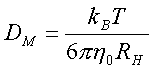 (3)
(3)
and knowing that viscosity of the solvent h0 changes
quite negligible by adding the salt, remarkable decreasing of DM (two times by adding ca.
0,13 mol / l NaCl) can be explained only by increasing the translational
hydrodynamic radius RH of
colloidal particles. It seems to be the initial stage of the phase separation:
the formation of spherical droplets begins by aggregation of larger particles.
Obviously, NaCl dose of 0,13 mol/l increases RH two times on average that does not reach the sedimentation
instability (no settling is observed).
From Figure 2 it is seen that in a rough evaluation
the value of ST is independent
of added NaCl dose. At the first glance, a notable dependence was expected
here: existing theoretical model of the Soret effect in ionic ferrofluid
accounts the double layer thickness D,
and D » 1 / I 1 / 2, Ref. [5].
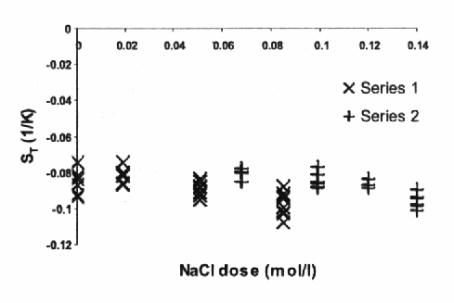
Figure 2. Measured Soret coefficient ST with adding NaCl.
Nevertheless, the situation is more complicated
because the theory predicts ST
to be proportional to RH [5].
Two effects: decrease of the double layer thickness D and increase of the
hydrodynamic radius RH by
amplifying the ionic strength really may compensate each other in studied range
of ionic strength 0 – 0,14 mol / l.
Conclusions.
Performed experiments indicate strong dependence of mass diffusion coefficient and no dependence of the Soret coefficient upon increasing the ionic strength. A correct theoretical explanation of these results leads to taking into account a lot of factors, e.g. interaction forces between particles and the particle size distribution, hence it seems to be rather complicated.
Authors are grateful to Dr. V. Cabuil for providing with an ionic ferrofluid sample, to Dr. V. Cabuil for providing with an ionic ferrofluid sample, to Dr E. Auzans for consultations in colloid chemistry and to Dr. K. I. Morozov for proposing experiments in this field.
References:
1. J. Lengl, Ph. D. Thesis, Univer. Paris 7, 1996.
2. A. Mezulis, Ph. D. Thesis, Univer. Paris 7, 1999.
3. A. Mezulis, E. Blums. A. Bourdon, G. Demouchy, Thermodiffusion-induced optical index grating in ferrocolloids, 4-th Int. Conf. PAMIR, France, 2000.
4. J. – C. Bacri, R. Perzinsky, D. Salin, V. Cabuil. R. Massart, Phase Diagram of an Ionic Magnetic Colloid // J. Colloid and Interfaces Science, 1989, V. 132, № 1.
5. K. I. Morozov // J. Magn. Magn. Mat., 201 (1999) 248.